Giant juvenile fibroadenoma: a systematic review with diagnostic and treatment recommendations
Introduction
Fibroadenomas are the most common type of breast tumors diagnosed in young women. Fibroadenomas found in children and adolescents are termed juvenile fibroadenomas (1). A juvenile fibroadenoma is considered “giant” if it is greater than 5 cm, 500 grams, or replaces at least 80% of the breast (1). Giant juvenile fibroadenomas are less common than fibroadenomas and comprise 1-8% of breast lesions in the adolescent population (1,2). Management of juvenile fibroadenomas includes surgical resection or observation since complete tumor regression may occur in 10-59% of lesions (1,3). These benign tumors have a propensity for rapid growth resulting in discomfort, self-consciousness, and anxiety (4,5). This is known to result in unpleasant interactions with their peers and has a considerable impact on these patients’ psychological and emotional state (4). Other conditions of the breast tissue may be mistaken for fibroadenomas including physiologic hypertrophy, phyllodes tumor, and inflammatory processes such as a breast abscess (6).
To date, there is a lack of specific guidelines regarding the optimal management of giant juvenile fibroadenomas likely due to conflicting diagnostic and treatment strategies, variation in patient age and the degree of breast development, and differences in patient preferences (7). The purpose of this study was to systematically review the available literature pertaining to giant juvenile fibroadenomas, to report data pertaining to the patient population and their clinical presentation, and to evaluate diagnostic and treatment strategies.
Materials and methods
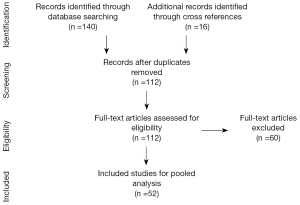
An electronic search of the MEDLINE and PubMed databases was conducted according to PRISM criteria to identify articles published between January 1946 and February 2014 (Figure 1) (8). Search terms included ‘fibroadenoma’, ‘juvenile fibroadenoma’, ‘giant’, ‘adolescent’ using controlled vocabulary (MeSH terms). Inclusion criteria included case reports and case series of giant juvenile fibroadenomas, written in English. Titles were reviewed by a single author (M.P) for candidacy of abstract review. Abstracts were then reviewed for full text article review. The bibliographies of all manuscripts were reviewed to identify additional references that were not captured in the initial search. Exclusion criteria included manuscripts focused on cytology/molecular analysis, those lacking clinical subjects, and those focused on non-giant juvenile fibroadenomas, animal studies, and review articles.
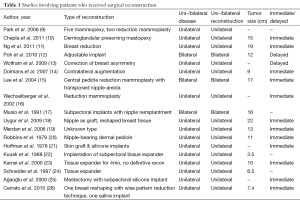
Data was then extracted from each manuscript to capture: patient age, comorbidities, tumor size (reported as largest dimension), presence of a single or multiple tumors, location, unilateral or bilateral, pain at presentation, time to intervention, diagnostic modality, treatment (observation, medical, or surgical), surgical complications, patient outcomes, time of follow-up, and whether patients underwent reconstructive surgery. The complete demographic and clinical data of the included studies are reported in Table 1.
Full table
Results
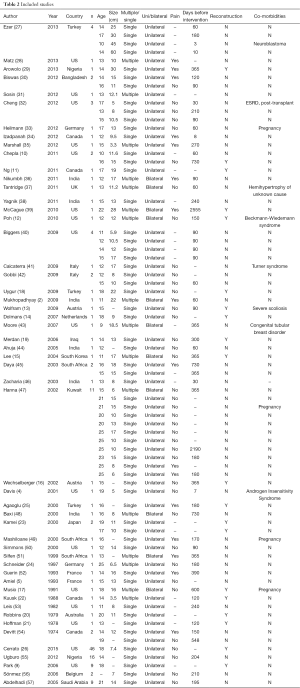
A total of 52 articles (47 case reports and 5 case series) met inclusion criteria and were included for review (Table 2). These articles encompassed a total of 153 patients with a mean age of 16.7±4.1 years (range, 9-25 years). Fifty percent of the articles (26/52), which included 103 patients, were published in the last ten years. Fibroadenoma size ranged from 3 to 60 cm with a mean of 11.2±9.09 cm. Eighty-six percent of cases (4,5,10,11,16,18,19,25,27,29,30,32-34,40-42,44-46,49,50,52,54) presented as a solitary mass (n=131) (9,13,14,19-21,23,26,38,47,53,55-57), while 14.4% presented with multiple masses (n=22) (2,12,15,17,22,24,28,31,35-37,39,43,47,48,51). Most cases (91.5%, n=140) presented as unilateral, while 8.5% (n=13) presented as bilateral. Follow-up time was recorded in 79.7% of patients (n=122). Mean follow-up was 14.7±18.8 months (range, 1 week to 84 months).
Full table
Pain was reported in 10.5% of cases (n=16). Imaging varied considerably with 72.5% of cases (n=111) having an ultrasound and 26.1% of cases (n=40) undergoing mammography. Of the patients who had a mammogram, 67.5% were cases that were published in the last 10 years. Tissue acquisition method included core needle biopsy in 18.3% (n=28), excisional biopsy in 11.1% (n=17), and fine-needle aspiration (FNA) in 25.5% (n=39) for cytological evaluation. Initial therapy included surgery in 98.7% of patients (n=151) and medical therapy in 1.3% (n=2). One patient received a gonadotropin-releasing hormone analog and medroxyprogesterone acetate, but the minimal clinical response ultimately led to surgery (51). A second patient was thought to have a breast mass as a result of an inflammatory process and was given antibiotics. A minimal response also led to surgical excision (42). Mean time to treatment was 9.5 months (range, 3 days to 7 years). Surgical intervention included mass excision in all cases with the exception of four patients (12,14,24,25) that required total mastectomy. Breast reconstruction was completed in 17.6% of cases (n=27). The type of breast reconstruction is detailed in Table 1. Most patients (92.2%) were in excellent health (n=141), while 5.2% (n=8) of patients had a pre-existing condition, including neuroblastoma (n=1) (27), (end stage renal disease) status post post-kidney transplant (n=1) (32), generalized body hemihypertrophy of unknown etiology (n=1) (37), Beckwith-Wiedemann syndrome (n=1) (12), Turner syndrome (n=1) (41), severe scoliosis (n=1) (13), congenital tubular breast disorder (n=1) (15), and androgen insensitivity syndrome (n=1) (4). Four patients (2.6%) were pregnant at the time of presentation (17,33,47,49). On final pathology, no specimen demonstrated malignancy. There were no reported post-operative complications. Fibroadenomas recurred in 3.9% of cases (n=6), requiring re-excision. Two patients demonstrated recurrence twice and the timing of recurrence ranged from 2 months to 4 years (9,22,37,41,51).
Discussion
Giant juvenile fibroadenomas, composed of epithelium and/or stroma of the terminal lobule of the breast, represent only 0.5% of all fibroadenomas (9,41). Fibroadenomas typically present as unilateral firm nontender masses that may enlarge with relation to the menstrual cycle (7). The term juvenile is a misnomer since giant fibroadenomas have been found in children as young as 9 years old and as old as 25 years old. In fact, a juvenile fibroadenoma has been reported in an infant as young as 3 weeks old (58).
Currently, there is a lack of clear guidelines regarding diagnostic and treatment modalities, and management varies among breast surgeons, obstetricians/gynecologists, pediatricians, and pediatric surgeons, all of whom may encounter a patient with a giant juvenile fibroadenoma. Referral to a specialist with experience in the management of such patients should facilitate a more focused evaluation and treatment strategy. The purpose of this literature review was to develop an evidence-based consensus regarding diagnosis and treatment by evaluating all reported cases of juvenile giant fibroadenomas. To our knowledge, this is the largest and most current clinical review of giant juvenile fibroadenomas.
Historically, fibroadenomas have been described as painless masses (59,60), yet 10% of the patients in our review reported pain. Over one third of the patients in this review underwent mammography in conjunction with another diagnostic modality, usually ultrasonography. The use of mammograms in young females has widely been documented to be of limited value due to increased breast density (60,61). The utility of mammography for a suspected giant juvenile fibroadenoma is limited due to poor image quality in younger patients as well as the extremely low risk of malignancy (42,57). Ultrasonography is the most common method of evaluation as demonstrated with this review. Smith et al. (62) found that patients aged 25 and younger suspected to have a fibroadenoma on ultrasound had 78.8% accuracy in diagnosis based on histology. However, amongst the same cohort of patients, ultrasonography proved to be a superior diagnostic negative predictive value for malignancy of 99.5% (42). Within that study, larger lesions, (3 cm or larger) and recurrent lesions were more likely to be diagnosed as phyllodes tumor or malignancy supporting the need to obtain tissue for diagnosis with large lesions. While FNA was used in over one third of the patients reported in this review, FNA may not reliably differentiate between a fibroadenoma and phyllodes tumor (11). In fact, one of the largest series of over 1,400 FNAs of adolescent breast masses, Kapila et al. concluded FNA is not required (63). The lack of an ideal diagnostic tool, coupled with the potential for rapid tumor growth makes complete surgical excision an excellent diagnostic and treatment modality. The safety profile of total excision is remarkable without reports of postoperative complications and a low recurrence rate of tumor.
Emphasis on preserving the developing breast parenchyma and nipple areolar complex is of paramount importance in achieving superior aesthetic results (61). Giant juvenile fibroadenomas may compress normal breast tissue, which may falsely minimize the perception of non-diseased parenchyma. However, the remaining displaced breast tissue will often fill in the void left by the excised giant juvenile fibroadenoma, precluding the need for reconstruction (31).
This systematic review did not disclose any malignancy or phyllodes tumor on final pathology. This may be due to the retrospective nature of this study. Most medical and surgical publications usually describe any giant mass in terms of the pathological disease on final pathology that would likely be missed on a database search. We address this limitation because of its importance in selecting proper diagnostic modalities during the fibroadenoma work-up.
According to the Surveillance, Epidemiology, and End Results (SEER) program maintained by the National Cancer Institute, the age-adjusted incidence of all malignant pediatric breast tumors in 2003 was 0.08 cases per 100,000 people and a total of 75 malignant breast tumors were identified over a 40-year interval (64). Because phyllodes tumors present in similar fashion and share histological similarities with fibroadenomas, surgeons may feel compelled to rule out malignant phyllodes tumor with a core needle biopsy as intervention would require wider excisional margins. However, the incidence of malignant phyllodes tumor is incredibly rare. Some of the largest reviews and case series describe 19 total cases reports in the literature up to 1994 (65), 5 cases between 1982 and 1996 (66), and 29 cases identified between 1973 and 2004 (64). Most childhood phyllodes tumors are benign (rarely borderline) and surgical intervention need not differ from simple excision (67-71). Based on the available phyllodes tumor data and this giant juvenile fibroadenoma review, it is reasonable to progress toward excisional biopsy in obtaining tissue for definitive diagnosis and as a form of intervention. Additionally, in our experience pediatric patients do not tolerate core needle biopsies well and the procedure may negatively impact the adolescent patient both psychologically and emotionally more than the adult patient. In the event that a patient defers excisional biopsy, tissue diagnosis using core needle biopsy (with/without ultrasound guidance or stereotactic techniques depending on lesion location) is appropriate. Furthermore, multiple conditions may predispose a young patient to malignant phyllodes tumor such as, childhood osteosarcoma (72), Hodgkins lymphoma (73), neurofibromatosis (74), and other genetic mutations prone to malignancy (Li Fraumeni spectrum syndromes, p53 mutation, BRCA1, BRCA2, etc.) (75). Core needle biopsy may be indicated in this high risk cohort.
Mastectomy as a treatment modality for giant fibroadenomas has been debated but is commonly reserved for unusual or recurrent cases (9). For the rare case requiring mastectomy as the initial form of excision, patients are likely to undergo reconstructive surgery. The majority of the patients who received breast reconstruction underwent immediate reconstruction, the advantages of which include limiting the treatment to a single surgical procedure and avoiding the psychosocial consequences of a breast deformity (9). However, the disadvantages of immediate reconstruction are two-fold, and include a compromised aesthetic result when the surgeon is unable to address minor revisions and when the surgeon is unable to achieve breast symmetry. Chang et al. (6) advocate for reconstruction according to three basic principles: “preserving all the normal breast parenchyma, adjusting the skin envelope, and positioning the nipple-areola complex for symmetry with the opposite breast.” The use of prosthetic implants in reconstruction, local dermoglandular rearrangement, reduction mammaplasty techniques, and nipple grafting have all demonstrated positive outcomes (9,10,12,15,25).
To date this is the largest and most comprehensive review of giant juvenile fibroadenoma, but there are limitations to the study. The major confounder to this systematic review is that only level four and five evidence (case reports and case series) are included that contribute to publication bias. These publications are often unique and include patients with large, unusual, or bilateral tumors. The incidence of patients with smaller tumors or tumors that did not warrant surgical treatment was less likely to be captured in our literature review. The lack of any postoperative complications is unusual but may be related to the youth and excellent health of the majority of patients. The option of observation as a management strategy is poorly described in the literature. However, the mean time to surgery was 9.5 months elucidating an increased likelihood of failed observation in most cases.
In summary and based on this systematic review, the authors recommend ultrasonography for lesion assessment and strongly encourage obtaining confirmatory tissue biopsy for histological evaluation. Tissue should be obtained based on patient-provider counseling and potential treatment strategy. If observation is recommended, a core needle biopsy should be considered (with/without ultrasonographic guidance or stereotactic guidance depending on the lesion location) to rule out malignancy. If surgical intervention is recommended (i.e., excision of lesion or mastectomy), a core needle biopsy or other invasive testing outside of the surgery should be avoided. There is little data to support the utility of mammography or FNA when evaluating a mass suspected to be a giant juvenile fibroadenoma.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Jayasinghe Y, Simmons PS. Fibroadenomas in adolescence. Curr Opin Obstet Gynecol 2009;21:402-6. [PubMed]
- Mukhopadhyay M, Patra R, Mondal S, et al. Bilateral giant juvenile fibroadenoma of breasts. J Indian Assoc Pediatr Surg 2009;14:68-9. [PubMed]
- Greenberg R, Skornick Y, Kaplan O. Management of breast fibroadenomas. J Gen Intern Med 1998;13:640-5. [PubMed]
- Davis SE, Wallace AM. A 19 year old with complete androgen insensitivity syndrome and juvenile fibroadenoma of the breast. Breast J 2001;7:430-3. [PubMed]
- Amiel C, Tramier D, Marck MF, et al. Giant breast fibroadenoma. J Gynecol Obstet Biol Reprod (Paris) 1993;22:764-5. [PubMed]
- Chang DS, McGrath MH. Management of benign tumors of the adolescent breast. Plast Reconstr Surg 2007;120:13e-19e. [PubMed]
- Cerrato F, Labow BI. Diagnosis and management of fibroadenomas in the adolescent breast. Semin Plast Surg 2013;27:23. [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [PubMed]
- Park CA, David LR, Argenta LC. Breast asymmetry: presentation of a giant fibroadenoma. Breast J 2006;12:451-61. [PubMed]
- Chepla KJ, Armijo BS, Ponsky TA, et al. Benefits of immediate dermoglandular preserving reconstruction following giant fibroadenoma excision in two patients. J Plast Reconstr Aesthet Surg 2011;64:e244-7. [PubMed]
- Ng WK, Mrad AM, Brown MH. Juvenile fibroadenoma of the breast: Treatment and literature review. Can J Plast Surg 2011;19:105-7. [PubMed]
- Poh MM, Ballard TN, Wendel JJ. Beckwith-Wiedemann syndrome and juvenile fibroadenoma: a case report. Ann Plast Surg 2010;64:803-6. [PubMed]
- Wolfram D, Behensky H, Piza-Katzer H. Unilateral gigantomastia related to juvenile fibroadenoma with idiopathic thoracic dcoliosis. J Pediatr Adolesc Gynecol 2009;22:e25-7. [PubMed]
- Dolmans GH, Hoogbergen MM, van Rappard JH. Giant fibroadenoma of one breast: Immediate bilateral reconstruction. J Plast Reconstr Aesthet Surg 2007;60:1156-7. [PubMed]
- Lee CJ, Kim YJ, Seo YT, et al. Treatment of multiple bilateral juvenile fibroadenomas in a teenage breast by central pedicle breast reduction, with vertical and short horizontal scar: case report. Aesthetic Plast Surg 2004;28:228-30. [PubMed]
- Wechselberger G, Schoeller T, Piza-Katzer H. Juvenile fibroadenoma of the breast. Surgery 2002;132:106-7. [PubMed]
- Musio F, Mozingo D, Otchy DP. Multiple, giant fibroadenoma. Am Surg 1991;57:438-41. [PubMed]
- Uygur F, Yigitler C. Rare juvenile giant fibroadenoma. J Breast Health 2009;5:164-6.
- Merdan I. Giant fibroadenoma: case report and review of the literature. Bas J Surg 2006;12:16.
- Robbins TH. Giant fibroadenoma of the breast: immediate reconstruction following excision using an inferiorly based nipple-bearing dermal pedicle. Br J Plast Surg 1979;32:292-4. [PubMed]
- Hoffman SH. Giant fibroadenoma of the breast: immediate reconstruction following excision. Br J Plast Surg 1978;31:170-2. [PubMed]
- Kuusk U. Multiple giant fibroadenomas in an adolescent female breast. Can J Surg 1988;31:133-4. [PubMed]
- Kamei Y, Torii S. Natural skin reduction and breast recovery using a tissue expander after enucleation of a giant breast tumour. Scand J Plast Reconstr Surg Hand Surg 2000;34:383-5. [PubMed]
- Schneider B, Laubenberger J, Kommoss F, et al. Multiple giant fibroadenomas: clinical presentation and radiologic findings. Gynecol Obstet Invest 1997;43:278-80. [PubMed]
- Ağaoğlu G, Ozgur F, Erk Y. Unilateral virginal breast hypertrophy. Ann Plast Surg 2000;45:451-3. [PubMed]
- Cerrato FE, Pruthi S, Boughey JC, et al. Intermediate and Long-term Outcomes of Giant Fibroadenoma Excision in Adolescent and Young Adult Patients. Breast J 2015;21:254-9. [PubMed]
- Ezer SS, Oguzkurt P, Ince E, et al. Surgical treatment of the solid breast masses in female adolescents. J Pediatr Adolesc Gynecol 2013;26:31-5. [PubMed]
- Matz D, Kerivan L, Reintgen M, et al. Breast preservation in women with giant juvenile fibroadenoma. Clin Breast Cancer 2013;13:219-22. [PubMed]
- Arowolo OA, Akinkuolie AA, Adisa AO, et al. Giant fibroadenoma presenting like fungating breast cancer in a Nigerian teenager. Afr Health Sci 2013;13:162-5. [PubMed]
- Biswas SK, Tanjihur Rahman AS, Paul AC, et al. Giant Juvenile Fibroadenoma of the Breast: Report of 2 Cases. Faridpur Med Coll J 2012;7:42-5.
- Sosin M, Feldman E. Giant juvenile fibroadenoma: a case and review of novel modalities in treatment. Breast Dis 2012;34:35-8. [PubMed]
- Cheng PJ, Vu LT, Cass DL, et al. Endoscopic specimen pouch technique for removal of giant fibroadenomas of the breast. J Pediatr Surg 2012;47:803-7. [PubMed]
- Heilmann T, Leuschner I, Hilpert F, et al. Diagnosis and management of an unilateral giant fibroadenoma of the breast in pregnancy. Arch Gynecol Obstet 2012;285:235-7. [PubMed]
- Izadpanah A, Karunanayake M, Izadpanah A, et al. An atypical growth of a giant fibroadenoma after trauma. J Pediatr Adolesc Gynecol 2012;25:e115-7. [PubMed]
- Marshall AP, Spottswood SE, Grau AM, et al. Juvenile fibroadenoma and granular cell tumor of the breast in an adolescent. J Pediatr Surg 2012;47:1930-3. [PubMed]
- Nikumbh DB, Desai SR, Madan PS, et al. Bilateral giant juvenile fibroadenomas of breasts:a case report. Patholog Res Int 2011;2011:482046.
- Tantrige PM, Hassanally D. Recurrent giant juvenile fibroadenomas with hemihypertrophy. Breast Dis 2011;33:41-4. [PubMed]
- Yagnik VD. Juvenile giant fibroadenoma. Clin Pract 2011;1:e49. [PubMed]
- McCague A, Davis JV. Giant fibroadenoma in a 22 year old patient: case report and literature review. Breast Dis 2010;31:49-52. [PubMed]
- Biggers BD, Lamont JP, Etufugh CN, et al. Inframammary approach for removal of giant juvenile fibroadenomas. J Am Coll Surg 2009;208:e1-4. [PubMed]
- Calcaterra V, Coscia DR, Sgarell A, et al. Recurrence of giant juvenile breast fibroadenoma in a girl with Turner’s syndrome. J Pediatr Endocrinol Metab 2009;22:281-3. [PubMed]
- Gobbi D, Dall’Igna P, Alaggio R, et al. Giant fibroadenoma of the breast in adolescents: report of 2 cases. J Pediatr Surg 2009;44:e39-41. [PubMed]
- Moore RL, Mungara A, Shayan K, et al. Bilaterally symmetric juvenile fibroadenomas and tubular breast deformity in a prepubescent girl. J Pediatr Surg 2007;42:1133-6. [PubMed]
- Ahuja A, Seth A. Juvenile fibroadenoma of Breast. Indian Pediatr 2005;42:72. [PubMed]
- Daya M, Mahomva O, Madaree A, et al. Reduction mammoplasty in cases of giant gibroadenoma among adolescent females. Case reports and literature review. S Afr J Surg 2003;41:39-43. [PubMed]
- Zacharia TT, Lakhar B, Ittoop A, et al. Giant fibroadenoma. Breast J 2003;9:53. [PubMed]
- Hanna RM, Ashebu SD. Giant fibroadenoma of the breast in an Arab population. Australas Radiol 2002;46:252-6. [PubMed]
- Baxi M, Agarwal A, Mishra A, et al. Multiple bilateral giant juvenile fibroadenomas of breast. Eur J Surg 2000;166:828-30. [PubMed]
- Mashiloane CD, Moodley J. Juvenile fibroadenoma during pregnancy. J Obstet Gynaecol 2000;20:86. [PubMed]
- Simmons RM, Cance WG, Iacicca MV. A Giant Juvenile Fibroadenoma in a 12-Year-Old Girl: A Case for Breast Conservation. Breast J 2000;6:418-20. [PubMed]
- Silfen R, Skoll PJ, Hudson DA. Florid juvenile (cellular) fibroadenomatosis in the adolescent: a case for subcutaneous mastectomy? Aesthetic Plast Surg 1999;23:413-5. [PubMed]
- Guerin C, Loget P, Watier E, et al. Giant juvenile fibroadenoma in an adolescent. A case report. Rev Fr Gynecol Obstet 1993;88:27-31. [PubMed]
- Leis HP Jr, Sabatini MT, Cleary JB, et al. Giant fibroadenoma of the breast in a prepubertal girl. Breast 1982;8:13.
- Devitt JE. Juvenile giant fibroadenoma of the breast. Can J Surg 1974;17:205-7. [PubMed]
- Ugburo AO, Olajide TO, Fadeyibi IO, et al. Differential diagnosis and management of giant fibroadenoma: comparing excision with reduction mammoplasty incision and rxcision with inframammary incision. J Plast Surg Hand Surg 2012;46:354-8. [PubMed]
- Sönmez K, Türkyilmaz Z, Karabulut R, et al. Surgical breast lesions in adolescent patients and a review of the literature. Acta Chir Belg 2006;106:400-4. [PubMed]
- Abdelhadi MS. Giant juvenile fibroadenoma: experience from a university hospital. J Family Community Med 2005;12:91-5. [PubMed]
- West KW, Rescorla FJ, Scherer LR 3rd, et al. Diagnosis and treatment of symptomatic breast masses in the pediatric population. J Pediatr Surg 1995;30:182-6. [PubMed]
- Fornage BD, Lorigan JG, Andry E. Fibroadenoma of the breast: sonographic appearance. Radiology 1989;172:671-5. [PubMed]
- Simmons PS. Diagnostic considerations in breast disorders of children and adolescents. Obstet Gynecol Clin North Am 1992;19:91-102. [PubMed]
- Templeman C, Hertweck SP. Breast disorders in the pediatric and adolescent patient. Obstet Gynecol Clin North Am 2000;27:19-34. [PubMed]
- Smith GE, Burrows P. Ultrasound diagnosis of fibroadenoma - is biopsy always necessary? Clin Radiol 2008;63:511-5; discussion 516-7. [PubMed]
- Kapila K, Pathan SK, Al-Mosawy FA, et al. Fine needle aspiration cytology of breast masses in children and adolescents: experience with 1404 aspirates. Acta Cytol 2008;52:681-6. [PubMed]
- Gutierrez JC, Housri N, Koniaris LG, et al. Malignant breast cancer in children: a review of 75 patients. J Surg Res 2008;147:182-8. [PubMed]
- Levêque J, Meunier B, Wattier E, et al. Malignant cystosarcomas phyllodes of the breast in adolescent females. Eur J Obstet Gynecol Reprod Biol 1994;54:197-203. [PubMed]
- Rajan PB, Cranor ML, Rosen PP. Cystosarcoma phyllodes in adolescent girls and young women: a study of 45 patients. Am J Surg Pathol 1998;22:64-9. [PubMed]
- Kaçar A, Paker I, Akbiyik F, et al. CD117 and CD34 staining patterns in childhood benign mammary lesions. Turk Patoloji Derg 2012;28:31-7. [PubMed]
- Selamzade M, Gidener C, Koyuncuoglu M, et al. Borderline phylloides tumor in an 11-year-old girl. Pediatr Surg Int 1999;15:427-8. [PubMed]
- Rodríguez Ogando A, Fernández López T, Rodríguez Castaño MJ, et al. Cystosarcoma phyllodes of the breast: a case report in a 12-year-old girl. Clin Transl Oncol 2010;12:704-6. [PubMed]
- Laufer J, Augarten A, Szeinberg A, et al. Pathological case of the month. Cystosarcoma phylloides in an adolescent female. Arch Pediatr Adolesc Med 1994;148:1067-8. [PubMed]
- Chung EM, Cube R, Hall GJ, et al. From the archives of the AFIP: breast masses in children and adolescents: radiologic-pathologic correlation. Radiographics 2009;29:907-31. [PubMed]
- Jaing TH, Yang CP, Hung IJ, et al. Phyllodes tumor in survivors of childhood osteosarcoma: a single institution’s experience. J Pediatr Hematol Oncol 2014;36:e36-8. [PubMed]
- Cucinotta M, Quartuccio N, Coppolino P, et al. A strange case of phyllodes tumor detected using (18)F-FDG PET/CT in an adolescent patient affected by Hodgkin lymphoma: a possible pitfall. Clin Lymphoma Myeloma Leuk 2014;14:e201-5. [PubMed]
- Jayasinghe Y, Simmons PS. Occurrence of two rare malignant neoplasms (breast and ovarian) in an adolescent female. J Pediatr Adolesc Gynecol 2009;22:e99-103. [PubMed]
- Bielack SS, Hecker-Nolting S, Kevric M, et al. More on osteosarcoma and phylloides tumor. J Pediatr Hematol Oncol 2015;37:158-9. [PubMed]