Current strategies with 2-staged prosthetic breast reconstruction
In conjunction with the passing of the Women’s Health and Cancer Right Act in 1998, and the increase in breast cancer awareness, the rates of breast reconstruction have increased dramatically. Nearly 1 in 8 women will develop breast cancer over her lifetime; an estimated 232,670 new cases of invasive breast cancer were expected to be diagnosed in women in the USA in 2014 (1). It was estimated that in 2014 alone, nearly 102,215 reconstructive procedures were performed for breast reconstruction (2). Post-mastectomy reconstruction has innumerable benefits to a woman’s sense of sexuality, body image, self-esteem and quality of life (3,4).
Breast reconstruction can be performed through a multitude of pathways: autologous (use of one’s own tissues), prosthetic (implant-based), or a hybrid of the two. The most common pathway for implant-based reconstruction is a 2-staged process where the first stage involves placement of a tissue expander and a second stage where the tissue expander is exchanged for a prosthetic breast implant (5). Nearly 70% of all breast reconstructions are prosthetic-based (6,7). Based on the Nationwide Inpatient Sample database from 1998 to 2008 there was an overall 78% increase in immediate breast reconstruction with a 203% rise in implant use (7). This trend continues today as advancements in technology continue to be made.
As oncologic principles and therapies have evolved so too have reconstructive tools and principles. Over the past 20 years, strides have been made in developing and refining tissue expanders, prosthetic breast implant devices, tools for intraoperative perfusion assessment, bioprosthetic materials for construction of internal support, and combining prosthetic reconstruction with autologous augmentation through fat grafting. All these advances allow reconstructive surgeons to take a once morbid and disfiguring procedure and make it a visually imperceptible defect.
Breast cancer management requires a multidisciplinary approach involving medical oncologists, radiation oncologists, pathologists, oncological surgeons and plastic surgeons. Ongoing communication amongst all parties involved during the planning stages allows for avoidance of potential postoperative complications and provides the best possible outcome for the patient. “A good reconstruction always begins with a good mastectomy” (8). It is imperative for reconstructive surgeon to be aware of the extent of resection and necessity for neoadjuvant and/or adjuvant therapies the patient may require such that the reconstructive timeline may be tailored to the individual patient.
Since introduction of the Halstead radical mastectomy in 1882, the extirpative surgery has evolved from a radical approach to a more conservative one where by the skin and/or nipple are spared (9,10). By maintaining the native breast envelope and inframammary fold, reconstruction of a natural, cosmetically appealing breast is possible at the time of mastectomy (11,12). While initial critics of the evolution raised concerns regarding compromising oncologic safety and potential increase in locoregional recurrence these well intentioned concerns have not been scientifically validated (13,14).
Once the glandular tissue has been removed, the reconstructive process commences. A 2-staged implant-based approach is begun through first placing a tissue expander to first save the natural breast footprint (inframammary fold, shape, width, and projection) and secondly to allow for expansion of the skin envelope to desired volume. At the second stage the expander is replaced with a long-lasting prosthetic device and refinements are made to the breast pocket and mound to achieve the desired aesthetic shape.
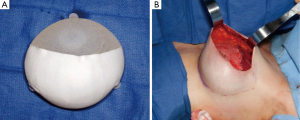
The concept of tissue expansion through placement of a subcutaneous balloon was first described by Neumann in 1957 to reconstruct an auricle (15). However, it was not until after 1982 when Radovan (16) published his experience with placing a deflated silicone expander with an external reservoir dome for reconstruction of the breast when 2-staged prosthetic breast reconstruction gained acceptance. The initial tissue expanders used by Radovan were round and dome-shaped with non-expandable bases and had external filling ports. The subsequent evolution included incorporation of the filling port into the device itself as to eliminate the need for dissection outside of the breast footprint and thereby reduce risks of lateral migration of the implant. In the late 1990s, McGhan Medical (Allergan) began production of variable height and variable projection devices which allowed for preferential expansion of the lower pole of the breast for a natural appearing breast (17). This was followed shortly thereafter with the incorporation of a textured surface and tabs to precisely control placement of the device and prevent any malpositioning and rotation of the device (18) (Figure 1). As tissue expanders have evolved so too have permanent prosthetic implants. Throughout history, there has been a desire for breast augmentation. In the 1800’s, there were reports of injecting various synthetic materials into breast including beeswax, petroleum jelly, and various epoxy resins (19). However, it was not until 1962 when Cronin and Gerow developed implants consisting of a thick silicone shell with a less viscous silicone filling which led to the modern era of breast implants. Unfortunately, in the 1970s the original generation implants had high failure rates with silicone leakage, high degree of capsular contracture and subsequent deformities which ultimately led to the temporary Federal Drug Administration’s (FDA’s) moratorium on silicone gel implants in 1992 (20). During the temporary embargo, silicone implants were utilized in clinical trials. Finally, in 2006, after multiple studies reported safety of the device, the FDA reversed the ban. Subsequent generations of breast implants have focused on creation of a higher fidelity shell to prevent silicone bleed, textured surfaces to prevent implant migration, cohesive silicone gels for a more natural feel, and anatomically shaped implants for a more natural appearance. Today’s implants, although made of similar material, are fundamentally different than previous generations.
While silicone implants have had a rocky history, saline-filled implants have remained on the market throughout the temporary silicone implant moratorium. However, these are not without faults. Initially described in France in 1965 by Arion, these devices were developed to allow for smaller incisions and versatility in adjusting volume and a soft, natural feel. Clinical trials in the 1970s showed high rates of deflation secondary to weak silicone shells and valve failures. Subsequent design modifications have resulted in deflation rates of 5.5% at 6 years (21). While saline breast implants are presently used in a fraction of primary cosmetic breast augmentation, they do not perform as well in the reconstructive realm; it is exceedingly difficult to achieve a natural appearing reconstruction with the use of saline implants. This is not just surgeon bias; this has been shown through patient reported outcomes. A study utilizing the BREAST-Q, a validated questionnaire measuring postsurgical body image and quality of life in the breast reconstruction, showed higher overall satisfaction with breast reconstruction, higher psychological well-being, higher sexual well-being, and higher satisfaction with surgeon for silicone implant recipients compared to saline implant recipients (22) (Figure 2).

The primary benefit of a 2-staged prosthetic approach is the placement of a partial deflated implant to preserve the breast footprint while not stressing the perfusion of the remaining mastectomy skin to prevent contracture of the wound while healing ensues. The nature of a mastectomy is inherently an ischemic process relative to the skin envelope. The perfusion of the breast arises from several sources including the internal mammary artery, lateral thoracic artery, thoracoacromial artery, and anterior/posterior branches of the intercostal arteries. The process of removing the glandular tissue eliminates the perfusion from the thoracoacromial artery and potentially from the other sources, particularly when the boundaries of the natural breast are violated. Skin necrosis, which was reported to occur in up to 25% of reconstructions, was plaguing complication early in the evolution of breast reconstruction, particularly in the immediate setting (23).
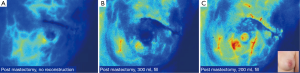
Accurate intraoperative prediction of skin flap viability with clinical judgement is a challenging task that often relies on subjective parameters including: color, capillary refill, and dermal edge bleeding. Assessment of skin flap perfusion with intraoperative LA-ICGA (laser-assisted indocyanine green fluorescent angiography) allows for real-time visualization of skin perfusion, providing the surgeon with an objective marker to facilitate surgical decision-making. The utility of LA-ICGA in predicting necrosis was illustrated in an article by Newman et al., in 2010 where LA-ICGA was performed on 20 consecutive mastectomy flaps showing a 95% correlation between intraoperative imaging and clinical course with 100% sensitivity and 91% specificity (24). A prospective trial of 51 implant based breast reconstruction LA-ICGA correctly predicted necrosis in 19 of 21 cases where clinical judgment failed (25). The Mayo Clinic adopted the technology in 2011 since has dropped the rate of skin necrosis in immediate breast reconstruction by 83% (26). Furthermore, when immediate implant-based reconstruction is to immediately follow the oncologic procedure, LA-ICGA allows for maximal fill volume without compromising perfusion of the mastectomy flap (Figure 3).
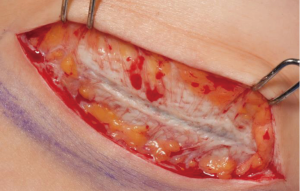
Traditionally, the prosthetic device has been placed in the sub-muscular plane with total submuscular coverage utilizing the pectoralis major and serratus anterior. The interposition of well vascularized muscular tissue between the skin and prosthetic device helped reduce the visibility of the implant under the skin and minimize the step-off between the device and chest wall (27). Conventionally, this involved elevation of the pectoralis major and serratus anterior fascia, however, this resulted in difficulty with inframammary fold definition, lateral deviation of the breast mound, failure to develop lower pole fullness, loss of a naturally ptotic appearing breast and a painful, prolonged expansion process. Furthermore, the submuscular pocket was taut while the overlying mastectomy was redundant resulting in contraction of the mastectomy flaps while the muscular pocket is slowly expanded. This resulted in disunion between the device and overlying skin envelope. These drawbacks of total submuscular coverage led to the use of acellular dermal matrices (ADMs) in breast reconstruction. ADMs are decellularized dermal matrices that provide a scaffold for the patient’s tissues to incorporate into through revascularization and repopulation. Breuing and Warren were the first to report use of ADM as an inferolateral dermal sling resulting in a partial subpectoral, partial sub-ADM pocket resulting in precise control of the lower pole and lateral mammary fold as well as reduced time to full expansion (28) (Figure 4).
ADMs have since revolutionized prosthetic breast reconstruction. Acting as internal support for the device, they provide precise control of the inframammary and lateral mammary folds, prevention of “window-shading” or retraction of the pectoralis muscle cephalad, shorter expansion times, reduction in implant visibility and rippling, and protective effects against radiation changes and capsular contracture (29-33). A diverse array of regenerative matrices are available; varying with respect to tissue source, processing, preparation, sizes, cost, and performance (34). ADMs have disrupted the dogma of total muscular coverage with the current technique of partial-muscular, partial-ADM coverage being routinely used. The door has now opened for total-ADM covered devices in the subcutaneous (pre-pectoral) plane. While the evolution from total muscular coverage to subcutaneous breast reconstruction is at the forefront of breast reconstruction with promising aesthetic outcomes, long-term results and complications are not yet available (27) (Table 1).
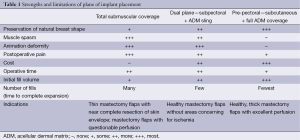
Full table
Historically, a consistent problem restricting the aesthetic outcome for prosthetic-based breast reconstruction was implant visibility and contour deformities; placement of an implant beneath an inherently thin skin envelope consistently generated an unnatural, conically shaped mound with obvious step-off between the implant and chest wall and lack of a naturally ptotic, tear-shaped breast. Currently, the solution to this problem is transplantation of fat from remote areas to the breast. This concept was first reported by Czerny in 1895 when he transplanted a lipoma to a breast after a partial mastectomy for fibrocystic disease (35). It was not until the 1980s with the advent of liposuction that fat grafting gained popularity as surgeons were now able to take a small aliquot of fat and inject it to fill contour deformities (36).
The general concept of modern fat grafting includes lipoaspiration at sites of excess adiposity (typically flanks, abdomen and/or thighs). This is done with a small 3- to 4-mm blunt cannula and negative pressure suction with a collection system between the suction device and cannula. The fat aspirated is then separated from the excess fluid and supernatant oils. The pure fat is then injected into the skin envelop in the subcutaneous plane between the dermis and underlying ADM capsule and/or muscle (Figure 5).
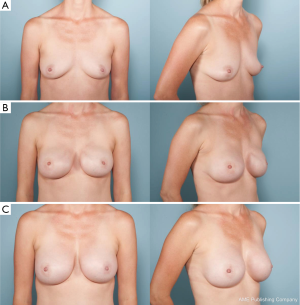
Early in the application of this technique to breast reconstruction, concerns were raised regarding not only the efficacy and long-term results but also oncologic safety. Science has yet to identify any association between autologous fat grafting and increased breast cancer recurrence (37-40). Furthermore, current studies have reported excellent aesthetic outcomes, a high degree of patient and surgeon satisfaction and overall a low rate of complications (38,41). More than just filling contour defects, autologous fat grafting fundamentally changes the quality of the overlying skin envelope especially in setting of radiation (42). Pre-clinical studies have shown reversal of radiation-induced dermal fibrosis and hypovascularity (43). Autologous fat grafting has proven to be a valued tool in breast reconstruction, which has revolutionized surgeons’ abilities to camouflage the prosthetic devices allowing for reconstruction of a natural breast.
Radiation therapy has become a mainstay in breast cancer treatment with more women being offer radiation treatment as studies have proven a survival benefit (44,45). This poses a challenge for reconstructive surgeons. Historically, prosthetic-based reconstruction was discouraged in the setting of post-mastectomy radiation due to the high rate of wound healing problems, implant malposition, capsular contracture, infection, extrusion of implants, and poor aesthetic outcome (46,47). However, with the adjuvant tools available including ADMs, anatomic breast implants, and fat grafting, successful prosthetic based reconstructions are now possible (48-51).
Breast reconstruction over the past decade has been completely revolutionized by the technical advances in oncologic management of breast cancer, development of anatomically shaped prosthetic devices, and application of bioprosthetic materials, intraoperative perfusion technology, and autologous fat grafting. Today’s breast reconstruction is nearly visually imperceptible, something that was a significant challenge with previous generations of technology, devices and techniques.
Acknowledgements
Authors’ contributions: Dr. Harless prepared the manuscript. Dr. Jacobson served as the principle investigator, responsible for the concept and oversight of the project in addition to refinement of the manuscript.
Disclosure: Dr. Jacobson is a consultant for LifeCell, Allergan, Sientra, and Mentor. Dr. Harless declares no conflict of interest.
References
- U.S. Breast Cancer Statistics. September 20, 2014. Available online: http://www.breastcancer.org/symptoms/understand_bc/statistics
- Surgeons ASoP. 2014 Reconstructive Plastic Surgery Statistics. 2014.
- Ng SK, Hare RM, Kuang RJ, et al. Breast Reconstruction Post Mastectomy: Patient Satisfaction and Decision Making. Ann Plast Surg 2014. [Epub ahead of print]. [PubMed]
- Howard-McNatt MM. Patients opting for breast reconstruction following mastectomy: an analysis of uptake rates and benefit. Breast Cancer (Dove Med Press) 2013;5:9-15. [PubMed]
- Serletti JM, Fosnot J, Nelson JA, et al. Breast reconstruction after breast cancer. Plast Reconstr Surg 2011;127:124e-35e. [PubMed]
- Wexelman B, Schwartz JA, Lee D, et al. Socioeconomic and geographic differences in immediate reconstruction after mastectomy in the United States. Breast J 2014;20:339-46. [PubMed]
- Albornoz CR, Bach PB, Mehrara BJ, et al. A paradigm shift in U.S. Breast reconstruction: increasing implant rates. Plast Reconstr Surg 2013;131:15-23. [PubMed]
- Nava MB, Ottolenghi J, Pennati A, et al. Skin/nipple sparing mastectomies and implant-based breast reconstruction in patients with large and ptotic breast: oncological and reconstructive results. Breast 2012;21:267-71. [PubMed]
- McLaughlin SA. Surgical management of the breast: breast conservation therapy and mastectomy. Surg Clin North Am 2013;93:411-28. [PubMed]
- Akram M, Siddiqui SA. Breast cancer management: past, present and evolving. Indian J Cancer 2012;49:277-82. [PubMed]
- Moyer HR, Ghazi B, Daniel JR, et al. Nipple-sparing mastectomy: technical aspects and aesthetic outcomes. Ann Plast Surg 2012;68:446-50. [PubMed]
- Chattopadhyay D, Gupta S, Jash PK, et al. Skin sparing mastectomy with preservation of nipple areola complex and immediate breast reconstruction in patients with breast cancer: a single centre prospective study. Plast Surg Int 2014;2014:589068.
- Lanitis S, Tekkis PP, Sgourakis G, et al. Comparison of skin-sparing mastectomy versus non-skin-sparing mastectomy for breast cancer: a meta-analysis of observational studies. Ann Surg 2010;251:632-9. [PubMed]
- Burdge EC, Yuen J, Hardee M, et al. Nipple skin-sparing mastectomy is feasible for advanced disease. Ann Surg Oncol 2013;20:3294-302. [PubMed]
- Neumann CG. The expansion of an area of skin by progressive distention of a subcutaneous balloon; use of the method for securing skin for subtotal reconstruction of the ear. Plast Reconstr Surg (1946) 1957;19:124-30. [PubMed]
- Radovan C. Breast reconstruction after mastectomy using the temporary expander. Plast Reconstr Surg 1982;69:195-208. [PubMed]
- Strock LL. Two-stage expander implant reconstruction: recent experience. Plast Reconstr Surg 2009;124:1429-36. [PubMed]
- Spear SL, Pelletiere CV. Immediate breast reconstruction in two stages using textured, integrated-valve tissue expanders and breast implants. Plast Reconstr Surg 2004;113:2098-103. [PubMed]
- Maxwell GP, Gabriel A. The evolution of breast implants. Clin Plast Surg 2009;36:1-13. [PubMed]
- Champaneria MC, Wong WW, Hill ME, et al. The evolution of breast reconstruction: a historical perspective. World J Surg 2012;36:730-42. [PubMed]
- Gutowski KA, Mesna GT, Cunningham BL. Saline-filled breast implants: a Plastic Surgery Educational Foundation multicenter outcomes study. Plast Reconstr Surg 1997;100:1019-27. [PubMed]
- Macadam SA, Ho AL, Cook EF Jr, et al. Patient satisfaction and health-related quality of life following breast reconstruction: patient-reported outcomes among saline and silicone implant recipients. Plast Reconstr Surg 2010;125:761-71. [PubMed]
- Jansen LA, Macadam SA. The use of AlloDerm in postmastectomy alloplastic breast reconstruction: part I. A systematic review. Plast Reconstr Surg 2011;127:2232-44. [PubMed]
- Newman MI, Samson MC, Tamburrino JF, et al. Intraoperative laser-assisted indocyanine green angiography for the evaluation of mastectomy flaps in immediate breast reconstruction. J Reconstr Microsurg 2010;26:487-92. [PubMed]
- Phillips BT, Lanier ST, Conkling N, et al. Intraoperative perfusion techniques can accurately predict mastectomy skin flap necrosis in breast reconstruction: Results of a prospective trial. Plast Reconstr Surg 2012;129:778e-88e. [PubMed]
- Harless CM, Jacobson SM. Tailoring through technology: A retrospective review of a single surgeon’s experience with implant-based breast reconstruction before and after implementation of Laser-Assisted Indocyanine Green Angiography. The Breast Journal 2015. (In Press).
- Reitsamer R, Peintinger F. Prepectoral implant placement and complete coverage with porcine acellular dermal matrix: a new technique for direct-to-implant breast reconstruction after nipple-sparing mastectomy. J Plast Reconstr Aesthet Surg 2015;68:162-7. [PubMed]
- Breuing KH, Warren SM. Immediate bilateral breast reconstruction with implants and inferolateral AlloDerm slings. Ann Plast Surg 2005;55:232-9. [PubMed]
- Nahabedian MY. AlloDerm performance in the setting of prosthetic breast surgery, infection, and irradiation. Plast Reconstr Surg 2009;124:1743-53. [PubMed]
- Breuing KH, Colwell AS. Immediate breast tissue expander-implant reconstruction with inferolateral AlloDerm hammock and postoperative radiation: a preliminary report. Eplasty 2009;9:e16. [PubMed]
- Gamboa-Bobadilla GM. Implant breast reconstruction using acellular dermal matrix. Ann Plast Surg 2006;56:22-5. [PubMed]
- Nahabedian MY, Spear SL. Acellular dermal matrix for secondary procedures following prosthetic breast reconstruction. Aesthet Surg J 2011;31:38S-50S. [PubMed]
- Spear SL, Seruya M, Clemens MW, et al. Acellular dermal matrix for the treatment and prevention of implant-associated breast deformities. Plast Reconstr Surg 2011;127:1047-58. [PubMed]
- Cheng A, Saint-Cyr M. Comparison of different ADM materials in breast surgery. Clin Plast Surg 2012;39:167-75. [PubMed]
- Czerny V. Drei plastische Operationen. III. Plastischer Ersatz der Brustdrüse durch ein Lipom. Arch F Klin Chir 1895;50:544-50.
- Mazzola RF, Mazzola IC. History of Fat Grafting: From Ram Fat to Stem Cells. Clin Plast Surg 2015;42:147-53. [PubMed]
- Pearl RA, Leedham SJ, Pacifico MD. The safety of autologous fat transfer in breast cancer: lessons from stem cell biology. J Plast Reconstr Aesthet Surg 2012;65:283-8. [PubMed]
- Agha RA, Fowler AJ, Herlin C, et al. Use of autologous fat grafting for breast reconstruction: a systematic review with meta-analysis of oncological outcomes. J Plast Reconstr Aesthet Surg 2015;68:143-61. [PubMed]
- Saint-Cyr M, Rojas K, Colohan S, et al. The role of fat grafting in reconstructive and cosmetic breast surgery: a review of the literature. J Reconstr Microsurg 2012;28:99-110. [PubMed]
- Gale K, Rakha E, Ball G, et al. A case controlled study of the oncological safety of fat grafting. Plast Reconstr Surg 2015. [Epub ahead of print]. [PubMed]
- de Blacam C, Momoh AO, Colakoglu S, et al. Evaluation of clinical outcomes and aesthetic results after autologous fat grafting for contour deformities of the reconstructed breast. Plast Reconstr Surg 2011;128:411e-418e. [PubMed]
- Rigotti G, Marchi A, Galie M, et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg 2007;119:1409-22; discussion 23-4. [PubMed]
- Garza RM, Paik KJ, Chung MT, et al. Studies in fat grafting: Part III. Fat grafting irradiated tissue--improved skin quality and decreased fat graft retention. Plast Reconstr Surg 2014;134:249-57. [PubMed]
- Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med 1997;337:949-55. [PubMed]
- Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;366:2087-106. [PubMed]
- Lee BT. Postmastectomy radiation therapy and breast reconstruction: an analysis of complications and patient satisfaction. Ann Plast Surg 2010;64:679-83. [PubMed]
- Alderman A, Gutowski K, Ahuja A, et al. ASPS clinical practice guideline summary on breast reconstruction with expanders and implants. Plast Reconstr Surg 2014;134:648e-55e. [PubMed]
- Clemens MW, Kronowitz SJ. Acellular dermal matrix in irradiated tissue expander/implant-based breast reconstruction: evidence-based review. Plast Reconstr Surg 2012;130:27S-34S. [PubMed]
- Lin KY, Blechman AB, Brenin DR. Implant-based, two-stage breast reconstruction in the setting of radiation injury: an outcome study. Plast Reconstr Surg 2012;129:817-23. [PubMed]
- Salgarello M, Visconti G, Barone-Adesi L. Fat grafting and breast reconstruction with implant: another option for irradiated breast cancer patients. Plast Reconstr Surg 2012;129:317-29. [PubMed]
- Cheng A, Lakhiani C, Saint-Cyr M. Treatment of capsular contracture using complete implant coverage by acellular dermal matrix: a novel technique. Plast Reconstr Surg 2013;132:519-29. [PubMed]


