Robotic transaxillary and retroauricular parathyroid surgery
Introduction
Robotic-assisted transaxillary parathyroidectomy using the da Vinci Si surgical system (Intuitive Surgical, Sunnyvale, CA, USA) has been described recently in several case reports and small series, as well as robotic-assisted retroauricular parathyroidectomy, which our initial experiences demonstrated as a safe and effective technique for parathyroid lesions (1-4). The robotic-assisted approach, among other reported routes, permits a safe, precise, 3-dimensional (3D) magnified dissection without the need for CO2 insufflation, and also has a better cosmetic result due to the invisible scar in the neutral position (5). Targeted parathyroidectomy has become the preferred procedure over bilateral neck exploration for primary hyperparathyroidism (PHPT) by most endocrine surgeons. When a parathyroid adenoma is localized preoperatively, it can be removed without visualizing the other glands. However, the indications will likely broaden as the procedure becomes more common. We and most parathyroid surgeons would definitively recommend against unilateral exploration or remote access techniques in patients with suspected multiglandular disease, since bilateral exploration can be performed safely through a very small cervical incision.
Parathyroidectomy for patients with PHPT is associated with a reported cure rate of 95-98% and a low rate (1-3%) of complications (6,7). Minimally invasive parathyroidectomy is being used more frequently, although it is generally not performed in patients with a large goiter or previous neck surgery (Table 1).

Full table
This review article will discuss some of the preoperative localizing imaging studies, intraoperative adjuncts and the two remote access approaches used for parathyroid surgery. An overview on our experience with the robotic-assisted transaxillary and retroauricular parathyroidectomy with the modifications required for the Western population will be discussed.
Preoperative localizing imaging studies
High-resolution ultrasound (US)
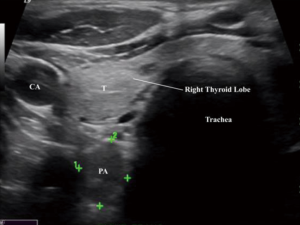
Of all the imaging modalities, US is the least expensive and least invasive, with no radiation or contrast exposure. A high-frequency linear transducer ideally in the range of 12-15 mHz is usually used. Parathyroid glands appear well circumscribed and most commonly oval in shape, but can be multilobed, elongated or bilobed. They are typically hypoechoic, in contrast to the hyperechoic thyroid follicular nodules and usually solid nodules (Figure 1). Other abnormalities include parathyroid cysts, which are usually thin walled structures with posterior enhancement and lacking internal echoes. Some of the factors that can limit the accuracy of US imaging are (I) operator skill and experience; (II) obesity, (III) smaller gland size; (IV) concurrent thyroid pathology (i.e., thyroiditis, multinodular goiter); (V) reoperative cases or previous neck surgery; (VI) retrotracheal, retroesophageal and mediastinal glands; and (VII) multiglandular disease. Although intrathyroidal parathyroid adenoma is a not a very common pathology, presenting in 1-3% of cases (8), a surgeon must always keep that possibility in mind, as it may warrant a thyroid lobectomy. The sensitivity of US in the detection of a parathyroid adenoma is very wide, ranging from 27% to 95% (9,10). One of US’s biggest disadvantages are that it is operator dependent, which most likely accounts for the wide range of the reported sensitivity. The combination of US and Sestamibi scan may increase the accuracy of localization of a single adenoma to 94-99% (10,11). The easy accessibility of US has led many surgeons to use it in the operating room prior to surgery to help identify the parathyroid adenoma and its exact anatomical location, which helps in precisely localizing the incision. Lastly, US-guided fine needle aspiration (FNA) can confirm intrathyroidal parathyroid adenomas in selected cases of persistent or recurrent hyperparathyroidism after failed exploration. An elevated parathyroid hormone (PTH) washout concentration from the FNA biopsy can help in identifying parathyroid gland lesions, which aids in performing a minimally invasive surgical approach even with a negative cytology, thus allowing the success of a targeted surgical approach in difficult cases (12).
Nuclear imaging
Currently, nuclear Tc-99m sestamibi (MIBI) scan is one of the most accurate and reliable means of preoperative parathyroid imaging. MIBI can find glands that are deep in the neck, posteriorly located or ectopic in the chest or mediastinum, many of which cannot otherwise be seen on US (Table 2). These abnormal parathyroid tissues are identified by detection of the mitochondrial uptake of the radionuclide tracer in areas that are hyperfunctioning. Currently there are no parathyroid specific radionuclides, hence all the radionuclides that accumulate in the parathyroid also accumulate in the thyroid glands. When the tracer is injected, a set of images is usually taken at 15 minutes and another delayed set after 2 hours. The tracer initially becomes concentrated in the normal thyroid as well as abnormal parathyroid tissues, which would be shown in the first set of images. The concentration of the tracer decreases rapidly in normal thyroid tissue, leaving behind foci of relatively enhanced uptake in abnormal parathyroid tissues, characterizing the hyperfunctioning glands which will be detected on the set of images taken 2 hours post tracer injection.
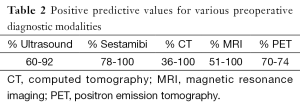
Full table
It must be kept in mind that a lack of retention of the tracer does not exclude the diagnosis of PHPT, as small adenomas and hyperplasia can be missed. Hence, utilizing MIBI and US in combination is recommended to localize parathyroid adenomas, a method reported to increase the sensitivity to 95% (11,13).
Computed tomography (CT)
CT is another noninvasive imaging study that is used to localize abnormal parathyroid glands. CT is usually utilized in localizing ectopic glands, such as those in the tracheoesophageal groove or anterior mediastinum (14). One of the advantages of CT is that it is less subjective than ultrasound. A more beneficial study that has been used is the four-dimensional computed tomography (4D-CT) scan.
4D-CT scan is similar to 3D-CT angiography except with the added dimension of changes in perfusion of contrast over time (15). 4D-CT has the ability to generate exquisitely detailed, multilane images of the neck, and allows the visualization of differences in the perfusion characteristics of hyperfunctioning parathyroid glands (i.e., rapid uptake and washout) compared with normal parathyroid glands and other structures in the neck. The 4D-CT is able to provide both anatomic and functional information in a single study that can be interpreted easily by the operating surgeon and serve an important role in localization before initial and/or re-operative parathyroid procedures. A recent study by Noureldine and Aygun et al. suggested that the 2-phase and 4-phase CT provide an equivalent diagnostic accuracy in localizing hyperfunctional parathyroid glands; in addition, the reduced radiation exposure to the patient may make 2-phase acquisitions a more acceptable alternative for preoperative localization (16).
Intraoperative adjuncts in parathyroid surgery
Radio guided parathyroidectomy (RGP)
RGP utilizes MIBI scan to help locate abnormal parathyroid glands prior to surgery. Patients are injected with the MIBI isotope approximately 1-2 hours prior to surgery. The gamma probe is then used to direct the incision site and localize the abnormal parathyroid glands during the surgery. After removal of the suspected adenoma, the gamma probe can be used again to confirm the high metabolic activity within the resected tissue as well as monitor the surgical bed to make sure no additional hyperactive glands are left behind. Some of the potential advantages of RGP include facilitation of a minimally invasive parathyroidectomy, shorter operating time, and the ability to verify a successful surgery. Absolute contraindications for RGP include pregnancy and allergy or sensitivity to MIBI.
Intraoperative PTH (IOPTH) assay
IOPTH monitoring has been advocated by some to minimize the possibility of persistent or recurrent hyperparathyroidism and improve the surgical success of minimally invasive parathyroidectomy. Demonstrating an appropriate reduction in IOPTH levels before the surgeon leaves the operating room, as well as assessing the adequacy of resection without the need to expose all the parathyroid glands, can confirm the patient’s eucalcemic status. Some of the advantages that IOPTH assay has brought to the field of parathyroid surgery are minimizing operative time, diminishing the need for bilateral neck exploration, and improving cure rates. IOPTH is based on the short half-life of circulating PTH. PTH is cleared from the blood in an early rapid phase with a half-life variously reported as 1.5-21.5 min in patients with normal renal function (17). The criteria for successful removal of hypersecreting glands are subject to ongoing debate, and the different approaches are summarized in Table 3. If the criteria are met, the operation is completed and the incision is closed. If the PTH fails to decrease sufficiently, further neck exploration is required. Several studies have aimed to explore the optimal percent decrease in PTH for the highest predictive cure rate. A decline of more than 50% in PTH level from the highest pre-excision level is associated with a predictive cure rate of 94-97% of cases. Some have used the return of PTH levels to the normal range as the optimal drop for a successful operation, but this criterion can be somewhat problematic since some patients have a slightly elevated baseline level. Different criteria may be utilized with similar accuracy rates. When used correctly, we believe that IOPTH is the most accurate adjunct available to the surgeon performing parathyroid surgery.

Full table
Methods
Indication and patient selection
The main concern many young females have when undergoing neck surgery, assuming there are no complications, is the incision length, location, design and healing in the assessment of the overall quality of the surgery. Therefore, guiding principles that can serve as a framework for the safe implementation of these emerging technologies in parathyroid surgery should be considered to avoid any unnecessary harm (Table 4). Nonetheless, ideal patient selection criteria are not well established. The ideal candidates for this approach are thin or average sized [body mass index (BMI) <30 kg/m2] young patients with concerns of a visible neck scar, or patients with a history of hypertrophic scar formation or keloids. With our extensive experience, we have been able to expand the selection criteria of our patients. Nevertheless, we still recommend being conservative with the selection criteria, especially during the beginning of the surgeon’s learning curve, which is vital for the safety and efficacy for robotic-assisted parathyroid procedures. This approach is usually deferred in patients with a previous history of neck surgery or irradiation of the neck. Patients should also be screened for contraindications that affect patient positioning during this procedure such as neck mobility problems and cervical spine disease.

Full table
Special equipment and room setup
One of the advantages of the robotic-assisted approach is its facilitation of an endoscopic neck surgery while maintaining a three-instrument approach. It also gives the surgeon the ability to retract, view target surgical anatomy, and still have two arms to operate, while maintaining traction and countertraction. The robotic wristed instruments permit the surgeon to reduce physiological tremors and increase the surgeon’s operative free dexterity of movement. Three robotic instruments (Maryland dissector, ProGrasp forceps and Harmonic curved shears) and a dual-channel camera are needed. By placing the camera through the axillary/retroauricular incision and using an endoscope with 30-degree down orientation, principles from the conventional cervical approach can be applied safely to this endoscopic technique. During development of the working space, electrocautery, a vascular DeBakey forceps and various retractors (army-navy, right-angled and lighted breast retractors) are used for subcutaneous flap dissection and elevation (Table 5).

Full table
It is important for the surgeon to determine the best way to organize the operating room prior to the procedure. The operating table should be positioned where the anesthesiologist has access to the patient’s airway. We highly recommend the use of a laryngeal nerve monitor. The patient cart is covered with sterile drapes and positioned on the contralateral side of the operating table. The patient cart is initially kept away from the operating table during the development of the working space to allow space for the surgical assistant to work across the table and retract the thyroid.
Operative technique, transaxillary approach
Step 1: patient positioning
It is essential that the patient is properly positioned for surgical exposure. Patients with limited shoulder range of motion are not good candidates for this approach. The arm and shoulder should be at the same vertical height, with proper padding of the forearm and elbow to prevent neuropraxia or stretch injury.
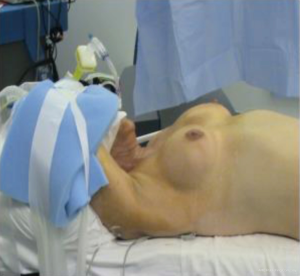
The patient is positioned supine under general anesthesia and intubated with an Nerve Integrity Monitor (NIM) endotracheal tube (Medtronic Xomed, Jacksonville, FL, USA) to allow intraoperative monitoring of recurrent laryngeal nerve (RLN) function. The neck is then slightly extended and the arm ipsilateral to the lesion (ipsilateral to the larger parathyroid gland) is placed cephalad and flexed above the head (modified Ikeda’s arm position) (Figure 2). Additionally, we routinely perform monitoring for the median and ulnar nerves using somatosensory evoked potentials (SSEP) (Biotronic, Ann Arbor, MI, USA) to avoid neuropraxia (Figure 3). However, many other robotic surgeons do not use SSEP and were able to avoid this serious complication by careful positioning of the arm. Other surgeons place the ipsilateral arm on an arm board. In our opinion, this would increase the dissection distance from the axilla to the thyroid bed. Professor Chung initially described rotating the ipsilateral arm to the lesion nearly 180 degrees cephalad, then placed on an arm board and padded. However, this was not very well tolerated in the Western population. Using this modified Ikeda’s arm position shortens the distance between the axilla and the thyroid space.
Intraoperative ultrasound examination is recommended by many robotic surgeons prior to the surgical incision, to further aid the surgeon in determining the exact location of the parathyroid lesion and to examine the relationship of the internal jugular vein to the thyroid gland in the anteroposterior plane.
During parathyroid surgery, Blood is drawn for a baseline rapid PTH serum level prior to prepping or palpating the neck.
Step 2: skin incisions
The location of the incision is determined by drawing a transverse line from the sternal notch laterally to the axilla, which marks the inferior limit of the incision. The inferior limit of the incision is directed posteriorly towards the patient’s back to ensure the incision will be well hidden. A 60-degree oblique line is drawn from the thyrohyoid membrane to the axilla, which marks the superior limit of the incision (Figure 4). The anterior chest and neck are prepped and draped. Following infiltration with 10 mL of 1% lidocaine with 1 in 200,000 adrenaline, a 5-6 cm incision is made with a scalpel from the intersection of the oblique line and the anterior axillary line as the superior limit and the intersection of the transverse line with the anterior axillary line as the inferior limit. Attention to detail in incising and handling the skin reduces cicatrix hypertrophy. For less experienced surgeons, the use of breast fold trocar will provide an easier operative technique. An additional small 0.8-cm skin incision can be made in the anterior chest wall, in the medial fold of the breast and 2-cm superior to the patient’s nipple. A trocar is used, and one of the robotic arms can be docked to the cannula and can assist in the manipulation, retraction and dissection of the thyroid gland. However, with experience, all instruments can be placed through a single axillary incision.
Step 3: establishing the working space
Dissection is then carried out using a monopolar electrocautery to create a subplatysmal plane anterior to the pectoralis fascia up to the clavicle. A wound protector (Alexis wound retractor system from Applied Medical, CA, USA) can be used to protect the axillary wound edges from any heat generated by the electrocautery or Harmonic scalpel and to expand the incision. Retractors can be used to maintain proper visualization as the dissection is carried out. An extended-tip Bovie is needed to aid in the dissection.
The clavicle is then identified and followed medially, which naturally leads to the dissection of the sternocleidomastoid (SCM) muscle (Figure 5). The surgeon should then develop a wide access to the midline of the neck through the axilla. This could be challenging in the obese population. When elevating the skin, the surgeon can avoid “buttonholes” into the skin by having an assistant pull the skin away from the tunnel. When using the lightened skin retractor, dissection should be carried out deep and lateral to the retractor to minimize the risk of skin injury. In general, the working space should be carried out from the clavicular head to just above the omohyoid muscle, which correlates with the superior pole of the thyroid lobe.
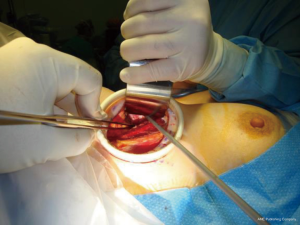
The triangulated window between the sternal head of the SCM (medially) and the clavicular head of the SCM (laterally) is then identified. Once these muscles are lifted, the surgeon will find the thyroid lobe covered by the adherent sternothyroid muscle. The uppermost fibers of the sternothyroid muscle are then dissected off the superior pole of the gland, as in open surgery. Using the Harmonic scalpel helps to develop a reasonable space between the sternal heads of the SCM, and the strap muscles are then lifted anteriorly by inserting and suspending the Chung retractor, creating the working space and exposing the anterior surface of the thyroid gland.
After suspending the retractor apparatus, the anesthesiologist should reconfirm adequate padding of the neck and shoulders. If the surgeon prefers, the second chest wall incision is made at this point. It is important to note that placing the chest wall trocar after the placement of the retractor apparatus will confirm the proper positioning of the chest wall retractor below the Chung retractor. However, placing the trocar prior to placing the Chung retractor would risk covering the entry of the chest wall retractor under the Chung retractor.
Operative technique, retro-auricular approach
Step1: patient positioning
Patients are placed supine on the operating room table. The head is turned to the side contralateral to the side of the diseased gland. Patients are intubated using a NIM endotracheal tube size 6.0 (Medtronic Xomed, Jacksonville, FL, USA) to allow intraoperative monitoring of RLN function.
Step 2: skin incisions
A small portion of postauricular hair is shaved for the extension of the planned incision lines into the hair-bearing skin. The retroauricular incision is then marked out just posterior to the earlobe, extending into the postauricular crease and adjacent to the occipital hairline at a position that will be covered completely by the ear and hair at rest.
Step 3: establishing the working space
The flap is created superficial to the platysma using a Metzenbaum scissor. Care is taken to preserve the greater auricular nerve. Dissection in the plane superficial to the platysma is performed until the head of the sternocleidomastoid muscle is visualized.
The space between the strap muscles and the SCM is created with electrocautery or Harmonic scalpel (Ethicon, Somerville, NJ, USA).
The working space is created all the way to just above the omohyoid muscle, which correlates with the superior pole of the thyroid lobe.
A specially designated retractor (Marina Medical, Sunrise, FL, USA) is then secured to the table mount lift and placed under the strap muscles to allow continuous exposure of the surgical field, maximizing access to the parathyroid gland. The flap creation time is approximately 30 minutes.
Step 4: docking of the da Vinci Si surgical robot
At this time, the da Vinci Si system (Intuitive Surgical, Inc., Sunnyvale, CA, USA) is docked using the 30° scope, Maryland dissector, and a Harmonic scalpel (The camera is positioned in the center of the field, a Maryland grasper is placed in the nondominant hand, and the Harmonic is placed in the dominant hand). Nerve dissection at the cricothyroid membrane entrance, and stimulation at its most consistent location just proximal to the inferior margins of the inferior constrictor muscle, is performed by the assistant at the bedside to confirm nerve integrity. The docking time is approximately 7 minutes.
Docking of the da Vinci Si surgical robot
The Intuitive Surgical Model Si robot (Intuitive, Sunnyvale, CA, USA) is docked from the contralateral side of the operative field, with the 30-degree down looking endoscope, Harmonic scalpel, and Maryland forceps entering via the axillary/Retro-auricular incision. For less experienced surgeons, the Harmonic curved shears are placed in the robotic arm corresponding to the surgeon’s dominant arm; however, this is not an issue with more experienced surgeons. Professor Chung recommends having the Harmonic scalpel in the patient’s “right-hand” position, regardless of the side of the lesion. Nevertheless, because the Harmonic scalpel does not have the same freedom of motion as other instruments, some surgeons move the Harmonic between the other robotic arms to improve visualization or the angle of approach. This maneuver is used more often when operating from the patient’s right-hand side. Currently, Intuitive has a vessel sealer with wristed movements which some surgeons are using during robotic thyroid surgery. However, this vessel sealer is significantly larger in size than the Harmonic Shears.
Placement of the instruments deserves special attention to allow optimal visualization and avoid collision of the instruments during the operation. The camera should be positioned high inside the wound, to provide a 30-degree downward view angle onto the thyroid bed.
Parathyroid dissection
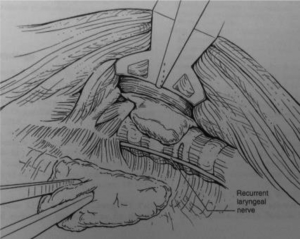
The army-navy retractor is used to retract the SCM clavicular head inferiorly. The thyroid gland is turned medially, then the plane between the parathyroid and the thyroid gland is identified and very meticulous circumferential dissection of the parathyroid from the thyroid gland is performed using a Maryland dissector. Adventitious tissue between the thyroid and parathyroid gland is dissected using the Harmonic scalpel. A gamma probe is brought into the field by the assistant and is used to confirm the location of the parathyroid gland. Identification of the inferior thyroid pedicle with dissection RLN in the tracheoesophageal groove is then undertaken to minimize the risk of injury to either structure (Figure 6). The nerve monitor stimulation is then brought to the field by the assistant to confirm the integrity of the RLN. After separating the parathyroid gland medially and circumferentially, the pedicle of the parathyroid is then identified and dissected at the base using the Harmonic scalpel. An endocatch bag is then brought into the field by the assistant, and the parathyroid gland is placed in the bag and removed safely through the retroauricular incision. A gamma probe is then used to confirm the absence of any residual parathyroid tissue.
Curative resection is established with the aid of IOPTH monitoring. We routinely place a drain protruding through the retroauricular incision. Interrupted subdermal closure is performed with 3-0 Vicryl suture. The skin at the hairline is closed with interrupted 5-0 Prolene sutures and staples (Figure 7). For patients who underwent concomitant neck lift surgery, the flap is created bilaterally using the above-mentioned approach. At the end of surgery and after the robot is undocked, excision of extra skin on both sides is performed prior to surgical site closure.
Postoperative management
Robotic-assisted transaxillary/retro-auricular parathyroidectomy is usually performed as an outpatient procedure. Patients are discharged on anti-inflammatory pain medication with narcotics only for breakthrough discomfort. Postoperative management is similar to that of the open approach. The drain is removed at the patient’s postoperative visit, usually 2 or 3 days after surgery. Due to the large working space, the risk of airway compression from hematoma postoperatively is lower when compared to that of the open approach. Therefore, some surgeons feel comfortable discharging their patients the same day of surgery with an advanced cold and compression regime, which helps with pain and swelling. Others will keep their patients overnight for observation. Many patients do not require any pain medication in their postoperative recovery.
Parathyroidectomy patients are supplemented with calcitriol 0.25 mcg twice daily and elemental calcium 1 g twice daily unless signs or symptoms of hypocalcemia present. No laboratory studies are required following intraoperative verification of serum PTH normalization. The patient’s first outpatient follow-up is 3-4 days postoperatively for pathology review, wound inspection and further instruction on wound care. Duration and extent of vitamin D and calcium supplementation are based on preoperative bone mineral density determination and interdisciplinary management with an endocrinologist.
Patients can be discharged on the same day of surgery.
Conclusions
Due in part to the social stigmatization of young females with visible scars, the remote endoscopic technique was implemented, refined and later enhanced with the advancement in robotic technology. The introduction of robotic technology into thyroid and parathyroid surgery has gained wide popularity in Asia, Europe and North American practice. Different approaches have been described recently, including axillary and the retroauricular region, resulting in a lack of visible neck scars. In order to maximize the utilization of these approaches, multiple preoperative scans must be performed in order to help localize the diseased gland for a targeted parathyroidectomy. Remote access has been shown to be a feasible and safe approach for parathyroidectomy in select patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Noureldine SI, Lewing N, Tufano RP, et al. The role of the robotic-assisted transaxillary gasless approach for the removal of parathyroid adenomas. ORL J Otorhinolaryngol Relat Spec 2014;76:19-24. [PubMed]
- Katz L, Abdel Khalek M, Crawford B, et al. Robotic-assisted transaxillary parathyroidectomy of an atypical adenoma. Minim Invasive Ther Allied Technol 2012;21:201-5. [PubMed]
- Landry CS, Grubbs EG, Morris GS, et al. Robot assisted transaxillary surgery (RATS) for the removal of thyroid and parathyroid glands. Surgery 2011;149:549-55. [PubMed]
- Foley CS, Agcaoglu O, Siperstein AE, et al. Robotic transaxillary endocrine surgery: a comparison with conventional open technique. Surg Endosc 2012;26:2259-66. [PubMed]
- Mohamed SE, Li X, Khadra H, et al. Different surgical approaches in parathyroid adenoma resections. Gland Surg 2013;2:227-9. [PubMed]
- Udelsman R, Donovan PI, Sokoll LJ. One hundred consecutive minimally invasive parathyroid explorations. Ann Surg 2000;232:331-9. [PubMed]
- Macfarlane DP, Yu N, Leese GP. Subclinical and asymptomatic parathyroid disease: implications of emerging data. Lancet Diabetes Endocrinol 2013;1:329-40. [PubMed]
- Temmim L, Sinowatz F, Hussein WI, et al. Intrathyroidal parathyroid carcinoma: a case report with clinical and histological findings. Diagn Pathol 2008;3:46. [PubMed]
- Roy M, Mazeh H, Chen H, et al. Incidence and localization of ectopic parathyroid adenomas in previously unexplored patients. World J Surg 2013;37:102-6. [PubMed]
- Liu ST, Li P, Feng L, et al. Diagnosis and surgical treatment of parathyroid neoplasms. Zhonghua Yi Xue Za Zhi 2013;93:2062-4. [PubMed]
- Lumachi F, Zucchetta P, Marzola MC, et al. Advantages of combined technetium-99m-sestamibi scintigraphy and high-resolution ultrasonography in parathyroid localization: comparative study in 91 patients with primary hyperparathyroidism. Eur J Endocrinol 2000;143:755-60. [PubMed]
- Abdelghani R, Noureldine S, Abbas A, et al. The diagnostic value of parathyroid hormone washout after fine-needle aspiration of suspicious cervical lesions in patients with hyperparathyroidism. Laryngoscope 2013;123:1310-3. [PubMed]
- Kasai ET, da Silva JW, Mandarim de Lacerda CA, et al. Parathyroid glands: combination of sestamibi-(99m)Tc scintigraphy and ultrasonography for demonstration of hyperplasic parathyroid glands. Rev Esp Med Nucl 2008;27:8-12. [PubMed]
- Ismail M, Maza S, Swierzy M, et al. Resection of ectopic mediastinal parathyroid glands with the da Vinci robotic system. Br J Surg 2010;97:337-43. [PubMed]
- Chazen JL, Gupta A, Dunning A, et al. Diagnostic accuracy of 4D-CT for parathyroid adenomas and hyperplasia. AJNR Am J Neuroradiol 2012;33:429-33. [PubMed]
- Noureldine SI, Aygun N, Walden MJ, et al. Multiphase computed tomography for localization of parathyroid disease in patients with primary hyperparathyroidism: How many phases do we really need? Surgery 2014;156:1300-6. [PubMed]
- Xing M, Westra WH, Tufano RP, et al. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab 2005;90:6373-9. [PubMed]