Anatomy relevant to conservative mastectomy
Introduction
The detailed surgical anatomy will be the breast was of almost no consequence during the Halsteadian era when the standard treatment was a radical mastectomy. The resurgence of interest in preservation of the skin and nipple with a view to optimizing aesthetic outcome, so called “conservative mastectomy”, has led researchers to attempt to build upon the seminal work of Sir Astley Cooper (1).
The anatomy of the breast, in particular the nipple, is highly relevant to surgeons considering conservative mastectomy. This paper will describe the clinical anatomy of the ducts as this pertains to the margins of a conservative mastectomy, but also the vascular anatomy of the breast skin and nipple as this has implications for the risk of ischaemic complications. An understanding of the anatomy, together with careful surgical technique may minimise these. We will briefly consider the nerve supply to the nipple and the arrangement of smooth muscle in of the nipple as these are relevant to residual function of the nipple after conservative mastectomy. While the detailed lobar anatomy of the breast (2-5) is of interest in optimising breast conservation it is not relevant in the case of mastectomy so will not be covered here.
Embryological development of the nipple and ducts
Paired mammary ridges, also known as milk lines develop on the ventral surface of the embryo. These extend from the axilla to the inguinal region, however much of each line atrophies leaving only the part overlying the pectoral region (6). The ectoderm is responsible for the formation of the ducts and alveoli and the mesenchyme is responsible for the connective tissue and the vasculature of the breast. The ectodermal thickening of the mammary primordium grows downwards into the dermis (7) producing solid cords of ectodermal cells growing within the underlying mesoderm. These buds become canalized and later form the lactiferous ducts and alveoli. When the foetus is near term the nipple becomes everted and ready to accept the lactiferous ducts. Developmental abnormalities in this process in a minority of foetuses result in congenital abnormalities such as amastia (absence of one or both breasts), athelia (absence of one or both nipples) and polythelia (more than two nipples).
Anatomy for skin-sparing mastectomy
From a surgical perspective, there is a clear compromise between completeness of excision of at-risk ducts and likelihood of damaging the blood supply of the skin and nipple. Thus skin-sparing mastectomy requires careful surgical technique, as described in subsequent chapters (on skin-sparing and skin-reducing mastectomy).
The development of the breast from ectoderm and mesenchyme may explain the presence of an “oncoplastic plane”, seen by surgeons between the subcutaneous fat, and the fat of the breast itself (see Figure 1). Named, like the discipline of oncoplastic surgery, to reflect the marriage of ablative oncological surgery, with aesthetic plastic surgery, this is the key to an oncologically-sound skin-sparing mastectomy.
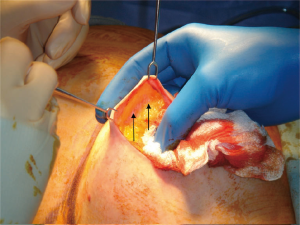
The breast tissue lies deep to this plane and the blood vessels, upon which the skin depends, run in the subdermal layer and are preserved with the skin, enhancing the aesthetic outcome of reconstruction. Failure to preserve the blood supply of the skin may result in necrosis of the skin flap, requiring debridement and possibly skin-grafting and risking infection and implant loss. Surgeons must, therefore, seek this plane, but in some patients it is easily found, and in others, more difficult. Anatomical (histological) studies shed some light on the reasons for this:
Beer et al. presented a histological study of thickness of the skin flap (i.e., depth of the oncoplastic plane) and showed great variability (8). Furthermore, they discovered that the fascial plane was not histologically distinguishable in 44% of resection specimens, and in some cases breast tissue came to within 0.4 mm of the surface of the skin. Larson et al. (9) also carried out histological examination of 76 breast specimens from 38 women undergoing reduction mammoplasty. The median subcutaneous tissue thickness (deep dermis to most superficial breast tissue) was 10 mm but with a wide range of 0-29 mm. The interquartile range was 6-17 mm. There was no correlation between the thickness of this subcutaneous tissue and body mass index, patient age, breast specimen weight, or dermis-to-breast thickness of the contralateral breast. Technical considerations (sampling and preservation of specimens) may partially explain these findings, but it is not uncommon, surgically, to find that the plane lies quite superficially in some patients and deeper in others, and indeed there may be variation within a patient in different quadrants. Hence no optimum mastectomy skin flap thickness can be recommended (10). Rather, the surgeon must be observant and careful when developing the plane.
Anatomy of the ducts
In addition to careful adherence to the oncoplastic plane, nipple-sparing mastectomy requires an understanding of the anatomy of ducts, their position within the nipple and their relationship to the vasculature and to the overall nipple shape. Again, surgical techniques for best managing this compromise will be discussed in later chapters. Here we present the relevant anatomy.
Number of ducts
In Sir Astley Cooper’s book “On the anatomy of the Breast”, he stated “The greatest number of lactiferous tubes I have been able to inject, has been twelve, and more frequently from seven to ten. But the greatest number of orifices I have been able to reckon has been twenty-two; however, some of these might be been follicles only, and not open ducts” (1). The variable results according to technique used, is reflected in the 21st century literature.
Going and Moffat (11) examined a single coronal section through the base of 72 nipples and found a median of 27 (IQR 21-30) collecting ducts. Similarly, Rusby et al. (12) studied 129 nipples and found the median number of ducts was 23 (IQR 19-28). Taneri et al. (13) sampled 226 mastectomy nipples histologically and found a mean of 17 (range, 18-30) ducts. Other techniques tend to result in smaller estimates of the number of ducts. For example, Ramsay et al. (14) used ultrasound to study 21 lactating women and found a mean of 9.6 ducts beneath the nipple of the left breast and 9.2 on the right. However, the equipment had insufficient resolution to identify ducts of less than 0.5 mm in diameter. Love and Barsky (15) employed several approaches to the study of ductal openings. Using serial sectioning and cytokeratin immunocytochemistry of ten nipples they identified 5-9 duct openings per nipple. They noted a mean of 5 duct openings by direct in vivo observations of lactating women and 6-8 openings by observation of passive conduction of lymphazurin from a subareolar injection to the nipple tip in mastectomy specimens. These findings are restricted to the number of ductal openings and do not establish the number of underlying ducts or their interconnections.
Relationship between ducts and openings
Four groups using histological techniques have noted the discrepancy between duct number and opening number and postulated that duct branching may be responsible (11,13,15). Going and Mohun (4) tried to elucidate the path of the 19 identifiable ducts in a 2.2 mm thick block at the tip of a nipple using episcopic fluorescence image capture (EFIC). However, they found that EFIC has insufficient resolution to discriminate reliably between keratin plugging and discontinuity between the duct and the skin surface. Using hematoxylin and eosin staining (H&E) sections from an entire nipple-tip, Rusby et al. showed that several ducts arose in the same cleft of the nipple (12), accounting for the discrepancy between the number of ducts in the nipple and the number of openings that can be counted externally.
Duct diameter
Estimating diameter at different levels has shown that most ducts are very narrow at the tip of the nipple with only a few ducts of a size that could be cannulated. At 1 and 1.5 mm beneath the tip the average duct diameter was 0.06 mm, and this increased to 0.7 mm at 3 mm deep (12).
Position of the ducts within the nipple
For conservative mastectomy, the exact number and size of the ducts is less relevant than their position and relationships to other structures in the nipple. The surgical community is divided over whether it is necessary to attempt to excise all of the ducts (potentially compromising blood supply) and it certainly might seem unnecessary to remove the duct core in prophylactic mastectomy since most tumours develop in the terminal ductal lobular units. However, it has been reported that 9-17% of nipples do contain lobular tissue (16,17), thus, potentially carrying the risk of de novo cancer formation within the nipple in high-risk women.
Duct arrangement is best seen in a three-dimensional image of a reconstructed nipple (12) (Figure 2).
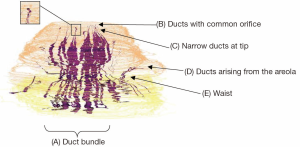
This shows:
- The ducts are arranged in a central bundle with a peripheral duct-free rim;
- The bundle narrows to a “waist” just beneath the skin, possibly at the level of the superficial fascia;
- Some ducts originate on the areola or part way up the nipple;
- Most ducts are very narrow as they approach the tip of the nipple;
- Many of the ducts originate within a smaller number of openings on the nipple surface.
The finding that the majority of ducts form a central bundle that occupies 21-67% of the cross-sectional area of the papilla (12) suggests that near-complete surgical excision of the central duct bundle is feasible if it is deemed advisable. The changing cross-sectional area of the duct bundle forms a “waist” as shown in the three-dimensional reconstructions (12,18). This may have a developmental origin as sagittal sections illustrate that the narrowest point of the duct bundle occurs at the level of the superficial fascia, perhaps indicating that in-growing ducts pierce this fascia together before dispersing into the developing breast. The waist may also correspond to the operative finding that the plane between breast and subcutaneous fat becomes more fibrous at the border of the nipple and this must be freed before the nipple can be inverted.
Going and Moffat (11) classified nipple ducts into three categories, ducts with a wide lumen, ducts with a minute lumen at the origin in the vicinity of the apex of the nipple and a minor duct population which arise from around the base of the papilla. Similar findings have been reproduced in other three-dimensional studies as well as identifying ducts originating in the areola (12). Going and Moffat’s hypothesis that larger ducts might be connected to larger duct systems were not confirmed in the aforementioned study by Rusby et al. as there was no organized relationship between size of duct and whether it terminated within the nipple or passed deeper into the breast.
Vascular anatomy of the nipple
Nipple necrosis after nipple-sparing mastectomy may result in a requirement for excision of the nipple. Nipple necrosis can also occur following surgery to correct inversion, for mammary duct fistula, and after Hadfield’s major duct excision. An understanding of the vascular anatomy is, therefore, clinically-relevant beyond nipple-sparing mastectomy.
Much of the available anatomical information about vascular anatomy within the breast and about supply to the nipple-areola complex is found in literature on breast reduction, where nipple viability is of key importance. Several studies have demonstrated that the blood supply of the breast is from the external and internal thoracic arteries, the intercostal, and the thoracoacromial arteries (19-22). Many of these studies were carried out in a small number of cadavers, which may account for discrepancies in comments on predominant supply to the nipple-areola complex.
Würinger (23) described two main sources of neurovascular supply to the nipple: a central and a superficial network. The central supply travels in a ligamentous septum originating from pectoralis fascia at the level of the 5th rib and inferior border of pectoralis major. Branches of the thoracoacromial, lateral thoracic and intercostal arteries and the deep branch of the 4th intercostal nerve passed within this septum. Würinger also described a medial ligament arising from the sternum and guiding blood vessels of the internal thoracic artery and anterior cutaneous intercostal nerve branches. A lateral ligament attached to the lateral border of pectoralis minor guides branches of the lateral thoracic and lateral cutaneous intercostal nerves. These ligaments merge and carry a blood supply to the superficial fascia.
O’Dey et al. (22) found that the lateral thoracic artery supplied up to three separate branches to the nipple-areola complex during its descending course. However, these passed through deep breast tissue before ascending towards the nipple-areola complex to reach the superolateral edge. While important in breast reduction, these branches would be divided during a mastectomy. O’Dey concluded that the internal thoracic artery, in particular, supplies the nipple-areola complex. 86% of cases studied had one or two perforating vessels usually emerging in the 2nd or 4th intercostal spaces. These vessels had a curved course with superior convexity and arrived at the supero-medial border of the nipple-areola complex. These are described as traversing the subcutaneous tissue, converging on the nipple-areola complex at a depth of 1.5±0.4 cm.
These studies all report that there is a superficial and a deep blood supply: the deep blood supply to the nipple shown in whole breast anatomical studies runs either through breast parenchyma (22) or in a ligamentous septum (24) and will be excised with the mastectomy specimen. If, according to O’Dey et al., the “superficial” supply runs approximately 1.5 cm deep to the skin surface it, too, is unlikely to be preserved during a good oncological mastectomy as it is unusual to leave skin flaps that are 1.5 cm thick (as described above). Furthermore, this implies that despite leaving 0.5 cm thickness of glandular tissue beneath the nipple as advocated by some surgeons, the most important vessels are likely to have been severed. Nakajima et al. (19) described branches of the external and internal mammary arteries travelling in the subcutaneous tissue and communicating with one another above and below the areola. Small branches derived from the communicating vessels were found running toward the nipple-areola complex. These small vessels reached the base of the nipple, giving off fine vessels to the areolar skin, and ascended in the nipple in a circular fashion. Nakajima found that these arborised in the upper and middle thirds of the nipple. The close proximity of these vessels to the ducts implies that any technique in which the nipple core is excised will result in disruption of the major neurovascular supply within the nipple. A subsidiary part of Nakajima’s work involved angiograms of breast skin specimens in which mammary glands and subcutaneous tissue had been resected. These showed rather sparse dermal and subdermal plexuses around the nipple-areola complex. It appears to be these plexuses upon which the survival of the nipple-areola complex depends if complete duct excision is attempted in nipple-sparing mastectomy.
Thus the two conflicting challenges of nipple preservation, ensuring oncological safety and maintaining nipple viability, are dependent on the underlying anatomy and on surgical technique and are inextricably linked through surgical judgment about the value of excising as much duct tissue as possible. Clinical series reporting necrosis rates often do not report in sufficient detail on surgical technique to allow readers to evaluate the trade-off being made.
Incision placement, however, is usually reported and many different incisions have been described for the conservative mastectomy with some high quality retrospective studies addressing this. A review of 48 studies by Munhoz et al. (25) demonstrated that the most common incision was the radial, followed by periareolar, inframmammary, mastopexy and transareaolar. Wijayanayagam et al. (18) found that the radial incision had the greatest likelihood of avoiding ischaemia of the nipple-areola complex in a series of 64 conservative mastectomies. However the scar from this incision is prominent. Colwell et al. (26) reviewed 500 nipple-sparing mastectomy procedures and found that a periareolar incision was an independent predictor of complications on multivariate analysis and the inferolateral inframammary fold incision was associated with a decreased risk of total and ischaemic complications. Similar results for the periareolar incision have been found in another study (27). Garwood et al. (28) found on logistic regression analysis that using an incision that was more than one third of the circumference of the nipple-areola complex was an independent risk factor for complete or partial nipple loss and skin flap necrosis. It can be assumed that if the sparse dermal and subdermal plexuses around the nipple-areola complex are disturbed in addition to division of the deeper vessels during the mastectomy, the risk of ischaemic complications is higher.
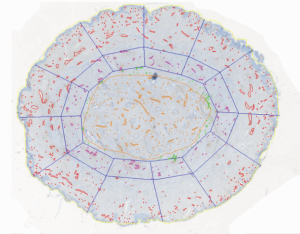
A study to investigate the microanatomy of the un-irradiated nipple vasculature used anti-factor VIII antibody to highlight blood vessels in sections from coronal 3 mm thick blocks of resected nipples. Within a 2 mm rim of peripheral nipple tissue 50% of the vessels were contained, and within a 3 mm rim, 66%. Only 29% of the vessels were located within the duct bundle (Figure 3). However, in terms of density, the mean microvascular density was 16 per mm2 in the duct bundle and 9 per mm2 in the peripheral tissue (29). The proportion of vessels in the duct bundle and the microvessel density was unchanged by radiation. These data are of anatomical interest, though it is difficult to apply these microscopic findings to improve surgical practice.
Anatomy of retained function
Opatt et al. (30) argue that sparing the nipple serves little purpose if the nipple is insensate. However, there is some evidence that nipple sensation and erection can be regained after nipple-sparing mastectomy (31-35).
The sensory innervation of the breasts comes from the lateral and anterior cutaneous branches of intercostal nerves (36,37). Controversies as to which intercostal nerves are relevant and their course are likely to be due to difficulty in dissecting thin nerves and the small number of cadavers in each study. Schlenz et al. (38) undertook an anatomic study of 28 female cadavers. They found that the nipple and areola were always innervated by the lateral and cutaneous branches of the 3rd, 4th and 5th intercostal nerves with the most constant innervation pattern being from the 4th lateral cutaneous branch. The anterior cutaneous branches took a superficial course within the subcutaneous tissues of the medial breast and terminated at the medial areolar border. The lateral cutaneous branches took a deep course within the pectoral fascia and reached the nipple via the breast parenchyma and pierced the nipple via its posterior surface. Montagne and Macpherson (39) demonstrated that the neural elements are concentrated at the base of the nipple with few at the side of the nipple and even fewer in the areolar. Therefore it is unsurprising that the nipple is largely insensate after nipple-sparing mastectomy due to injury of the anterior cutaneous nerves as the anatomical plane between the subcutaneous fat and breast parenchyma is developed and the lateral cutaneous nerves are divided as the breast parenchyma is separated from the pectoral fascia.
Although most authors report that sensation is lost, some preserved nipples remain erectile and therefore behave more naturally than a reconstructed nipple.
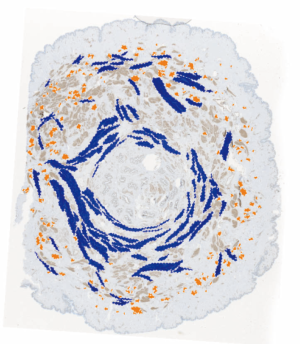
The arrangement of smooth muscle highlighted in Figure 4 (40) is reminiscent of the concentric muscle layers of the gastrointestinal tract or of a sphincter. At the base of the papilla the circular smooth muscle is particularly prominent around the duct bundle suggesting that contraction of this muscle could lead to erection of the nipple and possibly occlusion of the ducts. Conversely, towards the tip of the nipple, the concentrations of muscle fibres surround individual ducts as they narrow and unite close to the tip of the nipple.
Anatomy of lymphatic drainage
Sappey first described the anatomical basis of the breast lymphatics in the 1870s (41). He demonstrated a subareolar plexus of lymphatics and a small number of large lymphatic vessels draining into the axillary lymph nodes. Sappey concluded that the lymphatics of the breast collected in a subareolar plexus and then drained towards the axilla. Many of his observations contributed significantly to the development of breast lymphatic mapping and sentinel lymph node biopsy. In 1959 Turner-Warwick (42) studied the lymphatics and concluded that lymphatic pathways passed directly from the tumour injection site to the axillary lymph nodes without passing though the subareolar plexus. He suggested Sappey had mistaken mammary ducts for a lymphatic vessel, therefore overemphasizing the importance of the subareolar plexus. Whether or not the subareolar plexus drains the breast tissues and then lymph then drains towards the sentinel lymph node is still controversial and calls into question the optimal location of dye or radioisotope for sentinel lymph node biopsy. Suami et al. (43) undertook lymphatic mapping of 14 cadavers using hydrogen peroxide and injecting with a lead oxide mixture and then imaging the specimens. Similarly to Sappey they found the lymphatics deep to the nipple and areola were a dense network of lymph capillaries, however they favoured the Turner-Warwick findings that suggested a direct pathway from the injection site to the axilla, not via the subareolar plexus.
Conclusions
Together with careful surgical technique, a good working knowledge of the blood supply of the skin and nipple of the breast contributes to the avoidance of ischaemic complications in conservative mastectomy. Similarly, an understanding of the spatial relationships of ducts and blood vessels within the nipple will help surgeons make decisions on the relative benefits of removing or preserving the nipple core, and optimising technique to do so should this be deemed necessary.
Acknowledgements
The authors acknowledge the NIHR as the Royal Marsden and Institute for Cancer Research are an NIHR funded Biomedical Research Centre.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Cooper AP. editor. On the anatomy of the breast. London: Longman, 1840.
- Going JJ. Ductal-lobar organisation of human breast tissue, its relevance in disease and a research objective: vector mapping of parenchyma in complete breasts (the Astley Cooper project). Breast Cancer Res 2006;8:107. [PubMed]
- Tot T. The theory of the sick breast lobe and the possible consequences. Int J Surg Pathol 2007;15:369-75. [PubMed]
- Going JJ, Mohun TJ. Human breast duct anatomy, the 'sick lobe' hypothesis and intraductal approaches to breast cancer. Breast Cancer Res Treat 2006;97:285-91. [PubMed]
- Mannino M, Yarnold J. Effect of breast-duct anatomy and wound-healing responses on local tumour recurrence after primary surgery for early breast cancer. Lancet Oncol 2009;10:425-9. [PubMed]
- Seltzer V. The breast: embryology, development, and anatomy. Clin Obstet Gynecol 1994;37:879-80. [PubMed]
- Skandalakis JE, Colborn GL, Skandalakis PN, et al. Breast. In: Skandalakis JE. eds. Surgical Anatomy: The Embryologic and Anatomic Basis of Modern Surgery. Athens: Paschalidis Medical Publications 2004:155-88.
- Beer GM, Varga Z, Budi S, et al. Incidence of the superficial fascia and its relevance in skin-sparing mastectomy. Cancer 2002;94:1619-25. [PubMed]
- Larson DL, Basir Z, Bruce T. Is oncologic safety compatible with a predictably viable mastectomy skin flap? Plast Reconstr Surg 2011;127:27-33. [PubMed]
- Robertson SA, Rusby JE, Cutress RI. Determinants of optimal mastectomy skin flap thickness. Br J Surg 2014;101:899-911. [PubMed]
- Going JJ, Moffat DF. Escaping from Flatland: clinical and biological aspects of human mammary duct anatomy in three dimensions. J Pathol 2004;203:538-44. [PubMed]
- Rusby JE, Brachtel EF, Michaelson JS, et al. Breast duct anatomy in the human nipple: three-dimensional patterns and clinical implications. Breast Cancer Res Treat 2007;106:171-9. [PubMed]
- Taneri F, Kurukahvecioglu O, Akyurek N, et al. Microanatomy of milk ducts in the nipple. Eur Surg Res 2006;38:545-9. [PubMed]
- Ramsay DT, Kent JC, Hartmann RA, et al. Anatomy of the lactating human breast redefined with ultrasound imaging. J Anat 2005;206:525-34. [PubMed]
- Love SM, Barsky SH. Anatomy of the nipple and breast ducts revisited. Cancer 2004;101:1947-57. [PubMed]
- Rosen PP, Tench W. Lobules in the nipple. Frequency and significance for breast cancer treatment. Pathol Annu 1985;20:317-22. [PubMed]
- Stolier AJ, Wang J. Terminal duct lobular units are scarce in the nipple: implications for prophylactic nipple-sparing mastectomy: terminal duct lobular units in the nipple. Ann Surg Oncol 2008;15:438-42. [PubMed]
- Wijayanayagam A, Kumar AS, Foster RD, et al. Optimizing the total skin-sparing mastectomy. Arch Surg 2008;143:38-45; discussion 45. [PubMed]
- Nakajima H, Imanishi N, Aiso S. Arterial anatomy of the nipple-areola complex. Plast Reconstr Surg 1995;96:843-5. [PubMed]
- Ricbourg B. Applied anatomy of the breast: blood supply and innervation. Ann Chir Plast Esthet 1992;37:603-20. [PubMed]
- Wueringer E, Tschabitscher M. New aspects of the topographical anatomy of the mammary gland regarding its neurovascular supply along a regular ligamentous suspension. Eur J Morphol 2002;40:181-9. [PubMed]
- O'Dey Dm. Prescher A, Pallua N. Vascular reliability of nipple-areola complex-bearing pedicles: an anatomical microdissection study. Plast Reconstr Surg 2007;119:1167-77.
- Würinger E. Secondary reduction mammaplasty. Plast Reconstr Surg. 2002;109:812-4. [PubMed]
- Würinger E, Mader N, Posch E, et al. Nerve and vessel supplying ligamentous suspension of the mammary gland. Plast Reconstr Surg 1998;101:1486-93. [PubMed]
- Munhoz AM, Montag E, Filassi JR, et al. Immediate nipple-areola-sparing mastectomy reconstruction: An update on oncological and reconstruction techniques. World J Clin Oncol 2014;5:478-94. [PubMed]
- Colwell AS, Tessler O, Lin AM, et al. Breast reconstruction following nipple-sparing mastectomy: predictors of complications, reconstruction outcomes, and 5-year trends. Plast Reconstr Surg 2014;133:496-506. [PubMed]
- Regolo L, Ballardini B, Gallarotti E, et al. Nipple sparing mastectomy: an innovative skin incision for an alternative approach. Breast 2008;17:8-11. [PubMed]
- Garwood ER, Moore D, Ewing C, et al. Total skin-sparing mastectomy: complications and local recurrence rates in 2 cohorts of patients. Ann Surg 2009;249:26-32. [PubMed]
- Rusby JE, Brachtel EF, Taghian A, et al. George Peters Award. Microscopic anatomy within the nipple: implications for nipple-sparing mastectomy. Am J Surg 2007;194:433-7. [PubMed]
- Opatt D, Morrow M. The dual role of nipple preservation. J Support Oncol 2006;4:233-4. [PubMed]
- Petit JY, Veronesi U, Orecchia R, et al. Nipple sparing mastectomy with nipple areola intraoperative radiotherapy: one thousand and one cases of a five years experience at the European institute of oncology of Milan (EIO). Breast Cancer Res Treat 2009;117:333-8. [PubMed]
- Nahabedian MY, Tsangaris TN. Breast reconstruction following subcutaneous mastectomy for cancer: a critical appraisal of the nipple-areola complex. Plast Reconstr Surg 2006;117:1083-90. [PubMed]
- Denewer A, Farouk O. Can nipple-sparing mastectomy and immediate breast reconstruction with modified extended latissimus dorsi muscular flap improve the cosmetic and functional outcome among patients with breast carcinoma? World J Surg 2007;31:1169-77. [PubMed]
- Yueh JH, Houlihan MJ, Slavin SA, et al. Nipple-sparing mastectomy: evaluation of patient satisfaction, aesthetic results, and sensation. Ann Plast Surg 2009;62:586-90. [PubMed]
- Benediktsson KP, Perbeck L, Geigant E, et al. Touch sensibility in the breast after subcutaneous mastectomy and immediate reconstruction with a prosthesis. Br J Plast Surg 1997;50:443-9. [PubMed]
- Sarhadi NS, Shaw Dunn J, Lee FD, et al. An anatomical study of the nerve supply of the breast, including the nipple and areola. Br J Plast Surg 1996;49:156-64. [PubMed]
- Sarhadi NS, Shaw-Dunn J, Soutar DS. Nerve supply of the breast with special reference to the nipple and areola: Sir Astley Cooper revisited. Clin Anat 1997;10:283-8. [PubMed]
- Schlenz I, Kuzbari R, Gruber H, et al. The sensitivity of the nipple-areola complex: an anatomic study. Plast Reconstr Surg 2000;105:905-9. [PubMed]
- Montagna W, Macpherson EE. Proceedings: Some neglected aspects of the anatomy of human breasts. J Invest Dermatol 1974;63:10-6. [PubMed]
- Sappey MP. Anatomie, Physiologie, Pathologie des vaisseaux Lymphatiques consideres chez L’homme at les Vertebres. Paris: A. Delahaye and E. Lecrosnier, 1874.
- Turner-Warwick RT. The lymphatics of the breast. Br J Surg 1959;46:574-82. [PubMed]
- Suami H, Pan WR, Mann GB, et al. The lymphatic anatomy of the breast and its implications for sentinel lymph node biopsy: a human cadaver study. Ann Surg Oncol 2008;15:863-71. [PubMed]


