Evolution and update on current devices for prosthetic breast reconstruction
Introduction
The predominant modality for breast reconstruction has shifted from autologous to implant-based techniques with an over 2-fold increase since 1998 (1). As of 2010, 83% of breast reconstructions in the United States were performed with devices either in one or two stages (2). The reasons are multi-factorial, including greater awareness, overall patient preferences, changes in reimbursement, shorter operations and hospital stays and diminished relative contraindications for reconstruction in high-risk surgical and oncologic patients. One of the predominant reasons is increasing bilateral mastectomies (1,3). Qualitative studies point to physician recommendation, patient concern about recurrence, genetic susceptibility to breast cancer, increased use of breast magnetic resonance imaging, and desire for symmetry as the primary reasons women undergo bilateral mastectomy (4-6). Rise in implant-based reconstruction over the last decade is also concurrent with improvements in breast implant safety, quality, performance, and manufacturing. The Food and Drug Administration (FDA) has approved new implant styles, shapes and textures in just the last few years. As our choices in expanders and implants grow, so does our need for information surrounding safety, efficacy and outcomes data.
Modern generation breast implants can be divided into categories based on fill (saline versus silicone), shape (anatomic versus round) and surface structure (textured versus non-textured). Silicone gel implants can be further categorized by the degree and viscosity of gel fill and gel-shell interaction. Because each of these implant characteristics can affect feel and performance of the device, selection is dependent on the specific surgical indication along with patient and surgeon preferences. Various implant dimensions (height/width, projection and volume) allow individualization for each patient depending on the patient’s tissue quality/quantity and tissue-based bio-dimensional assessment. Breast device manufacturing and design spans several generations of refinements and advances in technology. The following review will journey through the evolution of various device characteristics leading up to the modern generation devices available today. We will further provide understanding into the safety and efficacy of current devices, highlighting the rigorous FDA hurdles surrounding their approval. We will discuss the advantages, disadvantages and indications for current generation device use as well as surgical advances that have enhanced device-based reconstruction.
Historic silicone gel devices
Silicone is a synthetic polymer made up of silicon, oxygen, carbon and hydrogen. The most common form is polydimethylsiloxane (PDMS), which contains a repeating SiO backbone with organic CH3 groups attached to the silicon atom (CH3)2SiO. Silicone fluids are composed of mostly PDMS straight chains. Silicone gels are polymeric networks of cross-linked PDMS swollen with silicone fluids. The extent of cross-linking and amount of fluid added to the gel accounts for the wide variety of viscosities and cohesivities of various generation silicone gel implants. Silicone elastomers that make up the implant shells are structured similar to gels but with much greater cross-linking, very little fluid and the addition of amorphous silica for strength. Barrier layer elastomers in modern generation implants contain either phenyl or trifluoropropyl to protect from gel bleed. Beneficial physical properties of silicone include stability across varying temperatures, low reactivity to other chemicals and low surface tension (7).
Since the introduction of silicone gel implants in the 1960s, their manufacturing and design have continued to evolve. Five main generations of silicone breast implants have been introduced to the United States market over the last 50 years (8). Originally implanted in 1962 for breast augmentation and reported by Cronin in 1963, the first generation silicone gel implants were introduced as new “natural feel” gel devices manufactured by Dow Corning Corporation (Midland, Michigan). A few years later, Cronin published his experience using these implants for single stage breast reconstruction after mastectomy (9). The initial design consisted of a thick elastomeric silicone outer shell (0.75 mm) and a thick, firm gel that together created an anatomically shaped device. Because the shell was smooth, Dacron (DuPont, USA) patches posteriorly were used to anchor the implant in situ. In 1969, Dow Corning began manufacturing the implants with a mandrel that was dip-coated which eliminated the peripheral seam (10). Capsular contractures were a common complication of these first generation devices, which were available from 1963 through 1972.
In an effort to create softer, natural feeling breasts, second-generation implants were developed with thinner more pliable shells and softer, less cohesive gels. The gel was composed mostly of low molecular weight chains instead of highly cross-linked silicone, which created a thin, less viscous gel. Contained in a shell only 0.2 mm thick, the thin silicone was able to diffuse across the intact shell causing silicone “bleed”. Despite gel bleed and shell failures, the second-generation implants were used into the mid 1980s. For thirteen years, breast implants were unregulated by the government. It wasn’t until 1976 that the FDA had authority to review and approve the safety and effectiveness data of new medical devices, including breast implants, under the Medical Devices Amendment to the Federal Food, Drug and Cosmetic Act. Existing devices, such as breast implants, were “grandfathered” in and allowed to remain on the market (11).
Concerns about silicone gel bleed, migration and possible systemic effects began to surface, so the third-generation silicone gel implants were designed to improve the shell strength and permeability. Multi-lumen implants were also introduced for the same reason, including the Becker implant, a permanent round expandable saline-gel device with a remote port (12). Previous silicone gel implant designs were improved by creating thicker silicone shells, up to 0.35 mm, and a protective barrier layer to prevent silicone gel bleed. Although the new designs were more durable with less shell failure (13), public concerns continued to escalate leading to classification of silicone gel implants as Class III devices by the US FDA in the 1980s. During this time, Dow Corning’s rat studies generated public warnings on the dangers of silicone implants and their possibility of causing cancer. Although the FDA panels could not find evidence to ban implants, they required pre-market approval (PMA) applications from all implant manufacturers. In addition, a national registry of women with breast implants was created to evaluate the possible association of implants with cancer and other systemic disease. In 1992, the FDA determined that the PMA applications for silicone gel implants were insufficient, citing the absence of data on safety and efficacy (14). By this time, Mentor Corporation and McGhan Medical Corporation were the only implant manufacturers who had not withdrawn from the US market. On January 5th, 1992, the US FDA announced a moratorium on the use of silicone gel filled breast implants with restricted use to participants in a clinical observational study, mostly for reconstructive purposes.
Current silicone gel devices
Despite access to silicone devices for breast reconstruction, many plastic surgeons switched to saline devices for all types of breast surgery during the silicone gel moratorium from 1992-2006. The smooth and textured round silicone gel fourth generation implants currently available today were developed in the early 1990s under strict quality, safety and performance standards. The new gel devices were filled with a more viscous, higher cross-linked gel and termed “cohesive”. In essence, all previous generations of silicone gel implants had some degree of cross-linking and therefore some degree of cohesion, but these devices were developed with more intended cross-linking than their predecessors. Both the fourth and fifth generation implants are generally referred to collectively as “cohesive implants”, manufactured with gel that is increasingly cohesive through these two generations correlating with increasing form stability and better maintenance of shape. Fourth generation round silicone gel implants were originally manufactured by Mentor Corp. (Santa Barbara, Calif) and McGhan/Inamed (now Allergan) Medical Corp (Santa Barbara, Calif). Both companies offer a portfolio of round smooth and textured devices in various widths and projections. Each manufacturer participated and submitted data from large-scale, prospective, multicenter trials evaluating preclinical safety and efficacy. In 2006, the US FDA approved marketing of implants from both Mentor (MemoryGel round implant) and Allergan (Natrelle round implant).
The Allergan 10-year Core Study, which began in 2000, is a prospective, multicenter, US FDA regulated clinical trial. Its purpose was to evaluate safety and efficacy of Natrelle round cohesive gel implants in women undergoing augmentation, reconstruction and revision surgery. Published results are available from both the 6- and 10-year data points. Of 715 subjects implanted with Natrelle round devices, 98 were post-mastectomy reconstruction patients and 15 were revision-reconstruction patients. At 10 years, 71.5% of reconstruction patients underwent reoperation most commonly for implant malposition followed by asymmetry. For all cohorts, the overall rupture rate was 7.7% for implants in subjects undergoing serial MRI. Capsular contracture rates were 24.6% for reconstruction and implant texture was not considered significant. Assessment of feel improved from 21.2% at baseline to 75.8% at 10 years with an overall satisfaction rate of 90.7%. Results of the core study demonstrate safety; efficacy and a high level of patient satisfaction with Natrelle round fourth generation silicone smooth and textured devices (15).
The Mentor 10-year Core Study, which began in 2000, is a prospective, multicenter, non-randomized, open label trial. Its purpose was to evaluate safety and efficacy of Mentor’s round silicone gel implants in women undergoing augmentation, reconstruction and revision surgery. Data from multiple time points have been published (16,17). Of 1,008 subjects, 251 patients were implanted at primary reconstruction and 60 patients were implanted at revision-reconstruction. The overall rupture rate for augmentation and reconstruction patients, including the MRI cohort, at 6 years was 2.6% for implants. However, when combined with the premarket approval longer-term data, implant rupture rate at 12 years was 9% (16), similar to the 7.7% rate at 10 years in the Allergan core study. Data from 6-year follow-up is the latest published time point to date. The Grade III/IV capsular contracture rate in primary breast reconstruction was 13.7%. Patient satisfaction with implant surgery was high with 97.8% of patients indicating they would have surgery again. In the reconstruction group, the re-operation rate for any reason was 33.9%, most commonly for asymmetry, followed by capsular contracture. Results of the core study established safety and efficacy of the Mentor MemoryGel implants. Further published reports are anticipated regarding the 10-year follow-up data (17).
The manufacturer-sponsored core studies adequately demonstrated safety as well as efficacy of the fourth generation round devices we use today. However, it’s important to realize that the core studies have many non-standardized variables in regards to surgeon skill, operative technique, post-operative management and adjuvant therapies. Therefore further investigation of long-term outcomes, specifically evaluating complications, reoperations and patient satisfaction with these devices is necessary. Capsular contracture is reportedly higher in reconstructive procedures compared to augmentation, and risk is progressively cumulative, increasing with time from implantation and just slightly less, although not significantly so, with textured devices (18). Future studies will need to re-evaluate these findings since the incidence of capsular contracture seems to be decreasing with use of biologics in first stage and revision reconstruction (19,20). Despite complications and re-operations, reconstructive patients with implants have high levels of satisfaction (18).
Fifth generation implants are generally considered cohesive form stable devices that retain their anatomic shape despite pressure from surrounding tissue. Most devices are textured to maintain proper positioning and orientation. The exception is Sientra’s round breast implant, which is the only FDA-approved (March, 2012) fifth generation round device, filled with high-strength cohesive (HSC) gel, available in both smooth and TRUE texture surfacing (21). All other fifth generation devices are shaped and textured. After 20 years of restricting use of shaped devices, the FDA-approved (March, 2012) Sientra’s High-Strength Cohesive (HSC+) filled device with TRUE texture surfacing. Shortly thereafter, in 2013, the US FDA approved marketing of both MemoryShape (Mentor, Santa Barbara, Calif.) and Natrelle 410 (Allergan, Irvine, Calif) form stable shaped devices.
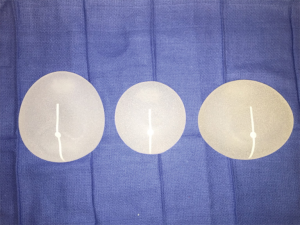
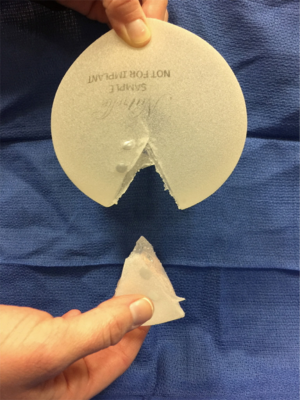
Sientra’s silicone gel breast implants are manufactured by Silimed and composed of a silicone elastomer shell with a barrier coat designed to minimize gel bleed. Every implant is filled with HSC silicone gel, a specifically formulated gel material manufactured by Applied Silicone Corporation (Santa Paula, Calif) and exclusive to Sientra’s breast implants (21). The Sientra fifth generation device portfolio includes round and shaped implants divided into categories based on profile, base shape and projection. The round devices are available in both smooth and TRUE texture surfacing. The smooth round devices have four different projection styles: moderate, moderate plus, moderate high and high whereas the textured devices are available in three different projection styles: low, moderate and high. Sientra offers five different styles of shaped form stable devices with three different base shapes (Figure 1): the classic-base moderate-projection, the round-base high projection, and the oval-base low, medium and high projection. The base shape is chosen based on the patient’s vertical and horizontal breast dimensions, taking into account the amount of projection needed. The classic base is used in women with vertically dominant dimensions, but does not offer as much projection as the other two available shapes. The round base is designed to optimize projection in women with similar vertical and horizontal breast measurements (Figure 2). The oval base can also optimize projection and provides increased breast width in reconstruction patients who have increased horizontal over vertical breast dimensions (22).
The Natrelle Style 410 matrix consists of 12 categories or cells of implants based on implant height (low-L, medium-M and full-F) and projection (low-L, medium-M, full-F and extra full-X). In February of 2013, the FDA approved four specific cells of Allergan’s form stable fifth generation silicone implants (Style 410 medium height, medium projection-MM, medium height, full projection-MF, full height, medium projection-FM and Style 410 full height, full projection-FF devices) for use in breast augmentation or reconstruction. The low and extra projection devices were only available to investigators in research studies through December of 2014, but were just approved by the FDA for unrestricted use in November of 2014. The wide variety of implant dimensions allows reconstruction of almost any breast footprint (Figures 3,4). The additional X projection devices provide patients with increased projection, even for larger volume breasts.
The Mentor MemoryShape breast implant was formerly known as the Contour Profile Gel or CPG device when used in U.S. research studies from 2000 to 2014. The only Mentor form stable device (MemoryShape) approved by the FDA in June of 2013 was the medium height, moderate projection implant. In September 2014, the FDA approved four additional styles of the Mentor MemoryShape devices: the low height, moderate plus projection implant, the medium height, moderate plus projection implant, the medium height, high projection implant and the tall height, moderate plus projection implant. Similar to Allergan devices, the Mentor MemoryShape devices offer a variety of sizes and are categorized based on their height (low, medium and high) and projection (moderate, moderate plus and high).
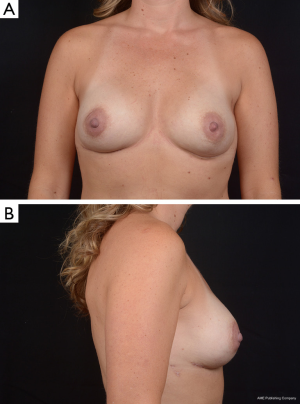
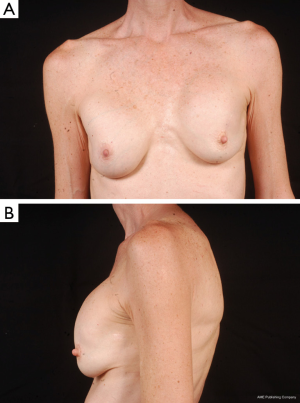
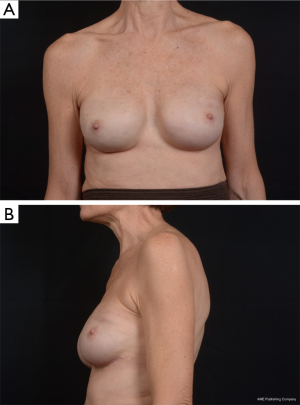
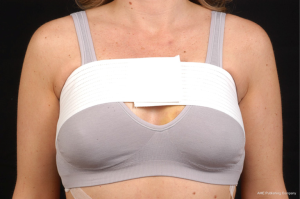
Use of shaped devices in breast reconstruction is safe and efficacious with predictable and reproducible results (23-26). Advantages include the ability to control breast shape, position and contour with good to excellent outcomes achievable in the majority of patients (22,27). Each manufacturer’s implant portfolio has characteristics that differ slightly but affect performance and satisfy a variety of patient desires and expectations. As for the degree of cohesivity, Allergan 410 implants are the most form stable, followed by Mentor MemoryShape implants and Sientra HSC devices, respectively (28). Increases in cross-linking and form stability correlate with increased shape retention but also increasing firmness of the device (Figure 5). However, firmness does not necessary correlate with increased strength, which is also dependent on gel/shell integration (28). Each form stable device is manufactured with circumferentially textured proprietary surfacing, differing in pore size to assist in positional stability and avoid rotation in the breast pocket. Reviewing studies with at least 5-year follow-up, capsular contracture and infection are low, ranging from 5-10% and 1-5%, respectively (24,25,29). The ability to avoid rotation with shaped devices is dependent on surgical technique with creation of a tight pocket, using capsulorrhaphies if necessary, and protocols such as judicious drain use and compressive bras or garments to prevent fluid accumulation in the periprosthetic space (Figure 6). Device malposition or rotation requiring reoperation ranges from 4-12%. Overall reoperation for any reason rates range from 43-45%.
Shaped vs. round silicone devices
Widespread consensus is lacking regarding the indications, advantages and disadvantages of shaped versus round silicone filled breast implants. Few studies have evaluated long-term performance and patient satisfaction comparing the two devices in breast reconstruction partly because shaped devices have only been on the US market a few years (30-32). Shaped devices have complication profiles similar to those of round implants and also have low rates of rotation in both aesthetic and reconstructive breast surgery (31). A recent study comparing round and shaped devices found lower rates of rupture and capsular contracture with shaped implants but the cumulative incidence of reoperation through nine years was similar (30).
In breast reconstruction, shaped implants can create a more naturally shaped breast mound with a gentle sloping upper pole and optimal lower pole breast projection. Because of the high cohesivity, the form stable devices tend to withstand deformational tensile forces (28) and are therefore a good option to correct deformities such as rippling or wrinkling. They can be especially useful in low body mass index patients or those with thin mastectomy flaps and deficient upper pole subcutaneous tissue. Technical considerations, such as precise breast pocket creation are paramount in avoiding rotation. For example, at the first stage of breast reconstruction, expansion is limited to avoid over-expansion of the pocket. Later, an equal or larger shaped device is placed with specific attention to pocket dimensions (Figure 7). This is in contrast to round gel implants that tolerate a larger pocket. Since rotation is not an issue, round implants may be more appropriate in difficult revision cases where many variables can affect the size and shape of the pocket. In general, round implants are felt to provide a softer, more natural breast feel. Patients will have movement of the implant within the breast pocket and are more likely to visualize and palpate wrinkling of the device. Therefore, the round devices are a good choice for women who have adequate upper pole tissue and who desire a soft natural feeling breast (30,31). A recent study detected no statistically significant difference in overall satisfaction with reconstruction when comparing shaped versus round silicone gel implants. Although patients reconstructed with the shaped devices reported firmer breasts, they were just as satisfied which could be because the implant chosen for each patient specifically suited the type of patient receiving it (32).
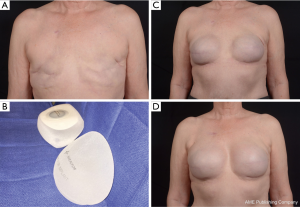
All manufacturers have a range of smooth and textured cohesive round and shaped implants with varying widths and projections. The recent additions of ultrahigh and extra projecting devices from each company have further increased options for reconstructive surgeons and allow creation of more projecting breast mounds (Figure 8). Breast reconstruction patients frequently rely on the expertise and advice of their surgeon when deciding on their final implant size and shape. The ability to convey various device characteristics and match them to the patient desires for feel and contour help surgeons chose the best device for each patient. Other factors to take into account include the upper breast pole soft tissue quality, bio-dimensional analysis, body mass index, and laterality of the reconstruction. Future outcome and satisfaction studies will continue to enhance our communication with patients and optimize our reconstructive results.
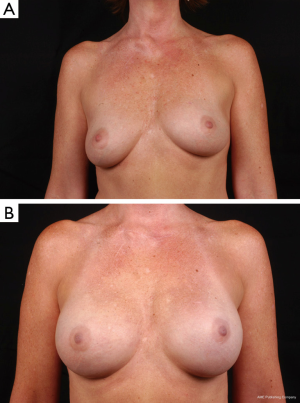
Inflatable breast implants: (saline)
Like the silicone gel filled implants, inflatable implants evolved through several generations of design and manufacturing. Only a few years after the first silicone gel breast augmentation in 1962, Dr. Henri Arion of France introduced the first inflatable breast implant. Over the next few decades, several renditions of inflatable designs were introduced, including the shaped saline device with optional Dacron patches. Unfortunately, these initial designs struggled with high spontaneous deflation rates due to seam and value issues, which were eventually solved with seamless, diaphragm-valve implants. Additionally, focus on appropriate fill volumes avoided the leaks from fold flaw cracking (33,34). The silicone moratorium in 1992 generated widespread use of saline filled breast implants for both breast augmentation and reconstruction. During this time of rigorous data collection for confirmation of safety and efficacy of devices, the US FDA examined evidence from both Mentor and McGhan Medical Corporation determining that saline-filled breast implants were safe and did not cause any major disease (35,36).
Few studies have evaluated the effect of implant fill type on patient perception of outcome after breast reconstruction. Overall patient satisfaction is high after breast reconstruction, whether they receive a silicone or saline implant (18). In 2010, Macadam studied the effect of saline versus silicone prosthetic breast reconstruction on patients’ postoperative satisfaction and found satisfaction was higher among those who received silicone implants compared with those who received saline implants (37). This finding was confirmed in a subsequent large multicenter cross sectional study (38).
Surface structure
The development of surface texturing resulted from discouraging high rates of capsular contracture with smooth walled implants in the 1960s. Ashley et al. published their initial experience using the first textured anatomic shaped silicone gel breast implant in 1970, which they developed and patented in 1968 (Natural-Y Prosthesis) (39). The texturing consisted of a 1 to 2 mm, fine cell polyurethane (PU) shell covering that allowed total tissue-implant fixation of the device. Several PU-coated implants were subsequently manufactured by different companies in a response to gaining popularity for the device’s ability to reduce capsular contracture rates (40,41). Early follow up of PU coated implants in immediate one stage breast reconstruction, even when placed subcutaneously, created soft, compressible breasts in most patients with low capsular contracture rates. The improved results were satisfying to both surgeons and patients (40). Reduced capsular contracture rates were due to in-growth of surrounding tissue into the fine cell PU, creating foreign body reaction. The chronic inflammation prevented circumferential linear fibrosis associated with the spherical contractile forces of capsular contracture (42,43). Unfortunately, the initial enthusiasm with early PU coated devices did not last at long term follow up as many women developed capsular contractures many years after implantation (44,45). In addition, explantation was difficult due to extensive in-growth of surrounding tissue (45). The delayed capsular contracture was thought to be due to progressive hydrolysis of the PU causing it to biodegrade, leaving behind a smooth walled implant. The uncoated device then acted as a smooth surface implant and likewise, developed capsular contracture at similar rates of other smooth wall devices of this era. One study reported the capsular contracture rate after implantation with PU coated devices at 6 to 10 years after implantation to be almost 60% (46). The situation worsened when animal studies linked one of the breakdown products of PU, 2,4-toluenediamine (TDA), to carcinogenesis (47). Therefore, in April 1991, PU coated implants were voluntarily removed from the US Market. Later, research concluded the lifetime risk of developing cancer from the PU metabolite, TDA, to be approximately one in one million and that there was no significant risk of cancer (48). Use of PU coated devices continued in several other countries with modifications including increased gel cohesivity and replacement of adhesive fixation with vulcanized thinner PU coating. Now once the PU disappears, the elastomere retains the imprint of the foam so the implant behaves as a textured device. Over 10-year long-term follow up of these devices (Silimed, Rio de Janeiro, Brazil) in 1,257 patients has revealed a very low capsular contracture rate of 1% (49).
Because the PU surface structure effectively decreased capsular contracture, there was strong enthusiasm to develop a similar textured silicone surface that would produce the same favorable response. The Biocell textured surface was designed in the late 1980s to promote tissue in-growth in an attempt to disrupt and prevent the circumferential linear fibrosis associated with capsular contracture around traditional smooth silicone surfaces (42). Each of the three current implant manufacturers retains proprietary texturing methods. The types of texturing include the Biocell surface texture by Allergan (Irvine, Calif), the Siltex surface texture by Mentor (Santa Barbara, Calif.) and the TRUE texture by Sientra (Santa Barbara, Calif.). Preventing peri-implant pathologic fibrosis was the original intent of new surface texture design. Indeed, each manufacturer’s textured devices share similar surface morphologies that disrupt regular capsule alignment and longitudinal contraction vectors resulting clinically in low capsular contracture rates (29,50-53).
Smooth surface implants are usually manufactured by repeatedly dipping mandrels in silicone and curing in a laminar flow oven. For textured implants, there is an intermediate step to allow texturing. The Biocell surface is manufactured by a “salt-loss” technique. Salt crystals are added to the dipped silicone mandrel before curing and then washed from the surface leaving behind a pitted appearance with randomly arranged cube indentations (53). The Siltex surface is created by pressing the dipped silicone mandrel into PU foam, a process termed negative contact imprinting. The resulting texture pore size, with a diameter of 70-150 µm (54), is meant to mimic the PU foam. TRUE texture is designed to promote tissue in-growth and is created neither by salt-loss, sugar, soak/scrub or imprinting, but a proprietary process that leaves behind smooth hollow pores with thin cell webbing that reduces particle formation (29,55).
Surface texture is an important implant characteristic for device stability, preventing rotation of form stable devices and migration of anatomic tissue expanders used in breast reconstruction. It has been postulated that the texture pore size correlates with tissue adherence and implant stability (54). Biocell texture with a pore diameter of 600-800 µm and depth of 150-200 µm (54) has been termed “aggressive” in that the capsule will grow into the pores creating a Velcro like effect between the device and the surrounding tissues (56). However, implant stability is also related to friction between the implant and the surrounding capsule, so despite the lack of tissue in-growth with Siltex, these form stable devices maintain proper position (56). Qualitatively, the TRUE texture is a hybrid of the other textures, more aggressive than Siltex but less aggressive than Biocell.
There are few disadvantages of textured breast implants when used in properly selected patients. Long-term outcomes studies show higher propensity of visible rippling and wrinkling and higher rates of saline implant deflation with textured devices (18). Double capsule formation, described as two layer capsular adherence, both to the device and to the adherent tissue, has been seen most commonly around textured devices but are of unknown clinical significance (57,58). Seromas may present as fluid collects between the two layers. These rare double capsules, reported at less than 1% in the literature (57), may form because of shear, trauma, infection, bio-films or large implant pockets limiting tissue-device adherence (58-61).
Safety of breast implants
Over the last several decades, breast implant safety has been studied more extensively than any other medical device. Concerns surrounding links to cancer, connective tissue disease and other systemic illnesses have been addressed in large epidemiological studies. In 1992, the same year of the silicone breast implant moratorium, two studies published evidence that women with implants are not at increased risk of developing cancer (62-64). Since this time, there has been overwhelming data confirming this claim as well as additional evidence that implants do not increase risk of recurrence when used in breast reconstruction nor do they cause non-breast tumors (65,66). Controlled epidemiologic studies have failed to find a causal association between silicone breast implants and connective tissue diseases or symptoms (67-69). The American College of Rheumatology released a statement endorsing the evidence and conclusions from these reports (70,71). In 1999, after a comprehensive assessment of silicone implants, the Institute of Medicine concluded that there was no evidence of a causal association between silicone-gel filled implants and connective tissue disease, rheumatic disease, neurological disease or cancer (72).
Implant associated anaplastic large cell lymphoma (ALCL) is a rare form of non-Hodgkin T cell lymphoma that has been reported in augmented and reconstructed women with saline and silicone implants. Primary lymphomas of the breast are rare and account for only 0.4-1% of all malignant breast neoplasms (73). Furthermore, ALCL only occurs in 0.1 per 100,000 women with or without implants (74). Although the US FDA in 2011 concluded there is a possible association between breast implants and ALCL, the rarity of the disease makes formulating epidemiologic studies and proving causality quite difficult. Extensive research is currently devoted to ALCL and its relationship to breast implants along with a registry of patients to facilitate data collection and a better understanding of the disease.
Tissue expanders
Although there is a place for single stage breast reconstruction with implants, two-stage implant based breast reconstruction using tissue expanders is currently the most commonly performed post-mastectomy breast reconstruction modality (75). A temporary device is placed at the time of mastectomy or at the first stage in a delayed breast reconstruction. After appropriate expansion and after adjuvant treatments, the expander is exchanged for a permanent implant.
The history of two-stage breast reconstruction dates back to the late 1970s when Birnbaum described two-stage breast reconstruction in a series of patients with an inflatable implant later exchanged for a custom silicone device (76). Around this same time, Radovan described using smooth walled temporary saline filled tissue expanders for breast reconstruction as an alternative to single stage silicone gel implant reconstruction (77). Expanders allowed for non-operative serial volume adjustments of the device to slowly stretch and mould the breast. Early expanders were burdened with complications such as infection, valve dysfunction, device failures, extrusion, malposition, capsular contracture, pain on expansion, and chest wall compression (78-80). However, many of these early concerns were alleviated with improvements in expander design as well as advances in surgical techniques.
Expanders were redesigned with integrated valves to decrease infection rates and resolve remote value issues such as valve flipping, pain and tube kinking (81). Capsular contractures around smooth expanders caused expander displacement and resistance to expansion with chest wall pain and compression. Biocell texturing of expanders created surrounding tissue adherence, which caused immobility of the device, but also reduced capsular contracture with progressive softening several weeks after expansion (42). Clinically, compliance allowed for further expansion with less pain and chest wall morbidity while immobility fostered ease of expansion in the desired location. At the time of tissue expander removal, the capsule and soft tissue cover were soft and pliable facilitating second stage reconstruction with a permanent implant without removal of the capsule (80). Anatomic shaped tissue expanders were introduced to produce a more natural breast appearance and to accommodate shaped devices at the second stage. The geometry of the device allowed for differential expansion, maximized in the lower pole of the breast (82,83).
The improved integrated-valve, textured, anatomic expanders produced low complication rates clinically. In 1998, Spear published his results using these devices in 171 immediate two-stage breast reconstructions with a Baker class III/IV capsular contracture rate of 3%, infection rate of 1.2%, overall deflation rate of 1.8% and no valve dysfunctions (81). Consistent, reproducible results were achieved with a 2004 follow up study with the same devices, but improved breast aesthetics due to change in device positioning from a total submuscular location to a partial subpectoral location allowing further lower pole expansion of the breast and accentuation of the inframammary fold, as well as a change from saline to silicone devices (84). Modern day tissue expanders are quite sophisticated with acceptable complication rates and high levels of overall patient satisfaction (85-88). In a recent report by Cordiero, 88% of patients had good to excellent aesthetic results following two-stage implant reconstruction (89).
The integrated-valve, textured, anatomic expanders are currently available today with the additional option of suture tabs (Figure 9). This optional refinement allows fixation of the device to the chest wall to further ensure stability, to prevent migration during expansion, to better control the anatomic boundaries of the breast pocket, thereby creating a more precise breast mound with less pocket modification at the second stage. With increasing use of acellular dermal matrix in the inferior breast pole at immediate breast reconstruction, the tabbed expander may allow for less variability and more reliance on the device to shape the breast mound. The outcome is more predictable at the second stage since the device is optimally placed on initial insertion with creation of more appropriate breast pocket dimensions (90).
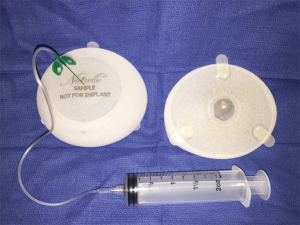
All three US implant manufacturers also have a portfolio of available tissue expanders with varying widths, heights, projections and volumes to match the patient’s bio-dimensional assessment. Many of the expanders are developed to match the corresponding manufacturer’s implant portfolio. Often, it is best to choose the desired implant first and then select an expander that is the same or smaller in dimensions than the anticipated implant. This is especially important for the shaped implants that require a precise pocket in order to avoid rotational deformity.
Sientra’s tissue expander product line consists of the ACX (Anatomical Controlled Tissue Expansion) matrix, which are either double chamber or single chamber (low, moderate and full height) devices, as well as round and crescent smooth and textured expanders with remote or integrated ports. The ACX devices have integrated ports, an orientation mark, TRUE texture surfacing and four suture tabs at the 4-, 8-, 10- and 2-o’clock positions for optimal stability. Sientra has the only double chamber breast tissue expander on the market and boasts differential expansion with optimal control.
Mentor’s Contour Profile (CPX) Expander portfolio consists of CPX3 and CPX4 devices. The previous CPX2 expander had an anatomic shape, SILTEX surface texturing, and an integrated injection dome with surrounding buffer zone with self-sealing technology and was available in low, medium and tall heights. The CPX3 expander matrix replaced this device and has all the same features of the CPX2 style with the addition of three suture tabs for stability at the 3-, 6-, and 9-o’clock positions. The slightly modified CPX4 tissue expander has a stronger magnet than the original contour profile device, an enhanced buffer zone self sealing-patch around the integrated port which is now flush without a palpable ring and a posterior Dacron patch to focus the expansion at the lower pole of the breast. Mentor also offers both a textured and smooth expandable implant with remote port (Spectra).
Allergan’s Natrelle 133 tissue expander is available in 84 sizes with variable projection in the low, short, moderate and full height devices as well as extra projection in the short, moderate and full height devices. Additionally, they are anatomically shaped, with integrated magnetic ports, Biocell texturing and are available with optional suture tabs at the 4-, 8- and 12-o’clock positions (Table 1).

Full table
Conclusions
Rates of implant-based reconstruction are increasing steadily. The current generation devices have been extensively studied and are deemed safe and efficacious with good aesthetic outcomes and acceptable complication and reoperation rates. Development of different styles of silicone gel implants, including more projecting devices and form-stable shaped devices increase choices for both surgeons and patients undergoing reconstruction. Introduction of acellular dermal matrices and improved surgical techniques further optimize reconstructive results. There is no one perfect implant, but with continuing research, development and long-term outcomes data, surgeons will be armed with the most up to date technology, along with the knowledge and expertise to provide the best possible prosthetic reconstructions.
Acknowledgments
Disclosure: The author is a speaker and consultant for Lifecell and Allergan; the author has equity ownership in Strathspey Crown Holdings, LLC (parent company of ALPHAEON Corporation).
References
- Albornoz CR, Bach PB, Mehrara BJ, et al. A paradigm shift in U.S. Breast reconstruction: increasing implant rates. Plast Reconstr Surg 2013;131:15-23. [PubMed]
- Gurunluoglu R, Gurunluoglu A, Williams SA, et al. Current trends in breast reconstruction: survey of American Society of Plastic Surgeons 2010. Ann Plast Surg 2013;70:103-10. [PubMed]
- Albornoz CR, Cordeiro PG, Farias-Eisner G, et al. Diminishing relative contraindications for immediate breast reconstruction. Plast Reconstr Surg 2014;134:363e-9e. [PubMed]
- Hawley ST, Jagsi R, Morrow M, et al. Social and Clinical Determinants of Contralateral Prophylactic Mastectomy. JAMA Surg 2014. [Epub ahead of print]. [PubMed]
- Han E, Johnson N, Glissmeyer M, et al. Increasing incidence of bilateral mastectomies: the patient perspective. Am J Surg 2011;201:615-8. [PubMed]
- Kummerow KL, Du L, Penson DF, et al. Nationwide trends in mastectomy for early-stage breast cancer. JAMA Surg 2015;150:9-16. [PubMed]
- LeVier RR, Harrison MC, Cook RR, et al. What is silicone? Plast Reconstr Surg 1993;92:163-7. [PubMed]
- Maxwell GP, Gabriel A. The evolution of breast implants. Plast Reconstr Surg 2014;134:12S-7S. [PubMed]
- Cronin TD. Subcutaneous mastectomy and gel implants. AORN J 1969;10:81-5. [PubMed]
- Cronin TD, Greenberg RL. Our experiences with the silastic gel breast prosthesis. Plast Reconstr Surg 1970;46:1-7. [PubMed]
- Freeman BS, Wiemer DR. Untoward results and complications following reconstruction after mastectomy. Clin Plast Surg 1979;6:93-105. [PubMed]
- Becker H. Breast reconstruction using an inflatable breast implant with detachable reservoir. Plast Reconstr Surg 1984;73:678-83. [PubMed]
- Yu LT, Latorre G, Marotta J, et al. In vitro measurement of silicone bleed from breast implants. Plast Reconstr Surg 1996;97:756-64. [PubMed]
- Kessler DA. The basis of the FDA's decision on breast implants. N Engl J Med 1992;326:1713-5. [PubMed]
- Spear SL, Murphy DK, Allergan Silicone Breast Implant USCCSG. Natrelle round silicone breast implants: Core Study results at 10 years. Plast Reconstr Surg 2014;133:1354-61. [PubMed]
- Cunningham B. The Mentor Core Study on Silicone MemoryGel Breast Implants. Plast Reconstr Surg 2007;120:19S-29S; discussion 30S-2S.
- Cunningham B, McCue J. Safety and effectiveness of Mentor's MemoryGel implants at 6 years. Aesthetic Plast Surg 2009;33:440-4. [PubMed]
- Handel N, Cordray T, Gutierrez J, et al. A long-term study of outcomes, complications, and patient satisfaction with breast implants. Plast Reconstr Surg 2006;117:757-67; discussion 68-72. [PubMed]
- Ho G, Nguyen TJ, Shahabi A, et al. A systematic review and meta-analysis of complications associated with acellular dermal matrix-assisted breast reconstruction. Ann Plast Surg 2012;68:346-56. [PubMed]
- Forsberg CG, Kelly DA, Wood BC, et al. Aesthetic outcomes of acellular dermal matrix in tissue expander/implant-based breast reconstruction. Ann Plast Surg 2014;72:S116-20. [PubMed]
- Calobrace MB, Capizzi PJ. The biology and evolution of cohesive gel and shaped implants. Plast Reconstr Surg 2014;134:6S-11S. [PubMed]
- Nahabedian MY. Algorithm and techniques for using Sientra's highly cohesive shaped silicone gel implants in primary and revision breast reconstruction. Plast Reconstr Surg 2014;134:28S-37S. [PubMed]
- Stevens WG, Harrington J, Alizadeh K, et al. Five-year follow-up data from the U.S. clinical trial for Sientra's U.S. Food and Drug Administration-approved Silimed(R) brand round and shaped implants with high-strength silicone gel. Plast Reconstr Surg 2012;130:973-81. [PubMed]
- Maxwell GP, Van Natta BW, Murphy DK, et al. Natrelle style 410 form-stable silicone breast implants: core study results at 6 years. Aesthet Surg J 2012;32:709-17. [PubMed]
- Hammond DC, Migliori MM, Caplin DA, et al. Mentor Contour Profile Gel implants: clinical outcomes at 6 years. Plast Reconstr Surg 2012;129:1381-91. [PubMed]
- Hedén P, Bronz G, Elberg JJ, et al. Long-term safety and effectiveness of style 410 highly cohesive silicone breast implants. Aesthetic Plast Surg 2009;33:430-6; discussion 437-8. [PubMed]
- Salgarello M, Farallo E. Immediate breast reconstruction with definitive anatomical implants after skin-sparing mastectomy. Br J Plast Surg 2005;58:216-22. [PubMed]
- Kinney BM, Jeffers LL, Ratliff GE, et al. Silicone gel breast implants: science and testing. Plast Reconstr Surg 2014;134:47S-56S. [PubMed]
- Stevens WG, Nahabedian MY, Calobrace MB, et al. Risk factor analysis for capsular contracture: a 5-year Sientra study analysis using round, smooth, and textured implants for breast augmentation. Plast Reconstr Surg 2013;132:1115-23. [PubMed]
- Caplin DA. Indications for the use of MemoryShape breast implants in aesthetic and reconstructive breast surgery: long-term clinical outcomes of shaped versus round silicone breast implants. Plast Reconstr Surg 2014;134:27S-37S. [PubMed]
- Nahabedian MY. Shaped versus Round Implants for Breast Reconstruction: Indications and Outcomes. Plast Reconstr Surg Glob Open 2014;2:e116. [PubMed]
- Macadam SA, Ho AL, Lennox PA, et al. Patient-reported satisfaction and health-related quality of life following breast reconstruction: a comparison of shaped cohesive gel and round cohesive gel implant recipients. Plast Reconstr Surg 2013;131:431-41. [PubMed]
- Rubin LR. The deflating saline implant--facing up to complications. Plast Reconstr Surg 1980;65:665. [PubMed]
- Kissin MW, Kark AE. Late leakage of saline-filled breast prosthesis. Arch Surg 1983;118:769. [PubMed]
- Rohrich RJ. The FDA approves saline-filled breast implants: what does this mean for our patients? Plast Reconstr Surg 2000;106:903-5. [PubMed]
- Cunningham BL, Lokeh A, Gutowski KA. Saline-filled breast implant safety and efficacy: a multicenter retrospective review. Plast Reconstr Surg 2000;105:2143-9; discussion 50-1. [PubMed]
- Macadam SA, Ho AL, Cook EF Jr, et al. Patient satisfaction and health-related quality of life following breast reconstruction: patient-reported outcomes among saline and silicone implant recipients. Plast Reconstr Surg 2010;125:761-71. [PubMed]
- McCarthy CM, Klassen AF, Cano SJ, et al. Patient satisfaction with postmastectomy breast reconstruction: a comparison of saline and silicone implants. Cancer 2010;116:5584-91. [PubMed]
- Ashley FL. A new type of breast prosthesis. Preliminary report. Plast Reconstr Surg 1970;45:421-4. [PubMed]
- Capozzi A, Pennisi VR. Clinical experience with polyurethane-covered gel-filled mammary prostheses. Plast Reconstr Surg 1981;68:512-20. [PubMed]
- Herman S. The Meme implant. Plast Reconstr Surg 1984;73:411-4. [PubMed]
- Barone FE, Perry L, Keller T, et al. The biomechanical and histopathologic effects of surface texturing with silicone and polyurethane in tissue implantation and expansion. Plast Reconstr Surg 1992;90:77-86. [PubMed]
- Brand KG. Foam-covered mammary implants. Clin Plast Surg 1988;15:533-9. [PubMed]
- Capozzi A. Long-term complications of polyurethane-covered breast implants. Plast Reconstr Surg 1991;88:458-61. [PubMed]
- Bruck HG. Long-term results of polyurethane-covered prostheses. Aesthetic Plast Surg 1990;14:85-6. [PubMed]
- Cohney BC, Cohney TB, Hearne VA. Nineteen years' experience with polyurethane foam-covered mammary prosthesis: a preliminary report. Ann Plast Surg 1991;27:27-30. [PubMed]
- Dunn KW, Hall PN, Khoo CT. Breast implant materials: sense and safety. Br J Plast Surg 1992;45:315-21. [PubMed]
- Hester TR Jr, Ford NF, Gale PJ, et al. Measurement of 2,4-toluenediamine in urine and serum samples from women with Meme or Replicon breast implants. Plast Reconstr Surg 1997;100:1291-8. [PubMed]
- Vázquez G, Pellón A. Polyurethane-coated silicone gel breast implants used for 18 years. Aesthetic Plast Surg 2007;31:330-6. [PubMed]
- Hakelius L, Ohlsen L. Tendency to capsular contracture around smooth and textured gel-filled silicone mammary implants: a five-year follow-up. Plast Reconstr Surg 1997;100:1566-9. [PubMed]
- Burkhardt BR, Eades E. The effect of Biocell texturing and povidone-iodine irrigation on capsular contracture around saline-inflatable breast implants. Plast Reconstr Surg 1995;96:1317-25. [PubMed]
- Malata CM, Feldberg L, Coleman DJ, et al. Textured or smooth implants for breast augmentation? Three year follow-up of a prospective randomised controlled trial. Br J Plast Surg 1997;50:99-105. [PubMed]
- Barr S, Bayat A. Breast implant surface development: perspectives on development and manufacture. Aesthet Surg J 2011;31:56-67. [PubMed]
- Danino AM, Basmacioglu P, Saito S, et al. Comparison of the capsular response to the Biocell RTV and Mentor 1600 Siltex breast implant surface texturing: a scanning electron microscopic study. Plast Reconstr Surg 2001;108:2047-52. [PubMed]
- Calobrace MB. The design and engineering of the MemoryShape breast implant. Plast Reconstr Surg 2014;134:10S-5S. [PubMed]
- Calobrace MB, Hammond D. Anatomic gel implants: from concept to device. Plast Reconstr Surg 2014;134:4S-9S. [PubMed]
- Maxwell GP, Brown MH, Oefelein MG, et al. Clinical considerations regarding the risks and benefits of textured surface implants and double capsule. Plast Reconstr Surg 2011;128:593-5. [PubMed]
- Toscani M, Rizzo MI, Spinelli G, et al. Breast implant complication: calcifications in the double capsule. Plast Reconstr Surg 2013;131:462e-4e. [PubMed]
- Góes JC, Landecker A. Optimizing outcomes in breast augmentation: seven years of experience with the subfascial plane. Aesthetic Plast Surg 2003;27:178-84. [PubMed]
- Pinchuk V, Tymofii O. Seroma as a late complication after breast augmentation. Aesthetic Plast Surg 2011;35:303-14. [PubMed]
- Hall-Findlay EJ. Breast implant complication review: double capsules and late seromas. Plast Reconstr Surg 2011;127:56-66. [PubMed]
- Deapen DM, Brody GS. Augmentation mammaplasty and breast cancer: a 5-year update of the Los Angeles study. Plast Reconstr Surg 1992;89:660-5. [PubMed]
- Berkel H, Birdsell DC, Jenkins H. Breast augmentation: a risk factor for breast cancer? N Engl J Med 1992;326:1649-53. [PubMed]
- Spear SL, Slack C, Howard MA. Postmastectomy reconstruction of the previously augmented breast: diagnosis, staging, methodology, and outcome. Plast Reconstr Surg 2001;107:1167-76. [PubMed]
- Kern KA, Flannery JT, Kuehn PG. Carcinogenic potential of silicone breast implants: a Connecticut statewide study. Plast Reconstr Surg 1997;100:737-47; discussion 48-9. [PubMed]
- Deapen D, Hamilton A, Bernstein L, et al. Breast cancer stage at diagnosis and survival among patients with prior breast implants. Plast Reconstr Surg 2000;105:535-40. [PubMed]
- Janowsky EC, Kupper LL, Hulka BS. Meta-analyses of the relation between silicone breast implants and the risk of connective-tissue diseases. N Engl J Med 2000;342:781-90. [PubMed]
- Tugwell P, Wells G, Peterson J, et al. Do silicone breast implants cause rheumatologic disorders? A systematic review for a court-appointed national science panel. Arthritis Rheum 2001;44:2477-84. [PubMed]
- Strom BL, Reidenberg MM, Freundlich B, et al. Breast silicone implants and risk of systemic lupus erythematosus. J Clin Epidemiol 1994;47:1211-4. [PubMed]
- Gabriel SE. Update on the epidemiology of the rheumatic diseases. Curr Opin Rheumatol 1996;8:96-100. [PubMed]
- Sánchez-Guerrero J, Colditz GA, Karlson EW, et al. Silicone breast implants and the risk of connective-tissue diseases and symptoms. N Engl J Med 1995;332:1666-70. [PubMed]
- Grigg M, Bondurant S, Ernster VL, et al. eds. Information for Women About the Safety of Silicone Breast Implants. Washington: National Academies Press (US), 2000.
- Domchek SM, Hecht JL, Fleming MD, et al. Lymphomas of the breast: primary and secondary involvement. Cancer 2002;94:6-13. [PubMed]
- de Jong D, Vasmel WL, de Boer JP, et al. Anaplastic large-cell lymphoma in women with breast implants. JAMA 2008;300:2030-5. [PubMed]
- American Society of Plastic Surgeons: Reconstructive Breast Surgery Statistics 2012. Available online: http://www.plasticsurgery.org/Documents/news-resources/statistics/2012-Plastic-Surgery-Statistics/Reconstructive-Surgery-Procedure-Trends-2012.pdf, accessed December 8, 2015.
- Birnbaum L, Olsen JA. Breast reconstruction following radical mastectomy, using custom designed implants. Plast Reconstr Surg 1978;61:355-63. [PubMed]
- Radovan C. Breast reconstruction after mastectomy using the temporary expander. Plast Reconstr Surg 1982;69:195-208. [PubMed]
- Slavin SA, Colen SR. Sixty consecutive breast reconstructions with the inflatable expander: a critical appraisal. Plast Reconstr Surg 1990;86:910-9. [PubMed]
- Francel TJ, Ryan JJ, Manson PN. Breast reconstruction utilizing implants: a local experience and comparison of three techniques. Plast Reconstr Surg 1993;92:786-94. [PubMed]
- Maxwell GP, Falcone PA. Eighty-four consecutive breast reconstructions using a textured silicone tissue expander. Plast Reconstr Surg 1992;89:1022-34; discussion 35-6. [PubMed]
- Spear SL, Majidian A. Immediate breast reconstruction in two stages using textured, integrated-valve tissue expanders and breast implants: a retrospective review of 171 consecutive breast reconstructions from 1989 to 1996. Plast Reconstr Surg 1998;101:53-63. [PubMed]
- McGeorge DD, Mahdi S, Tsekouras A. Breast reconstruction with anatomical expanders and implants: our early experience. Br J Plast Surg 1996;49:352-7. [PubMed]
- Eriksen C, Stark B. Early experience with the crescent expander in immediate and delayed breast reconstruction. Scand J Plast Reconstr Surg Hand Surg 2006;40:82-8. [PubMed]
- Spear SL, Pelletiere CV. Immediate breast reconstruction in two stages using textured, integrated-valve tissue expanders and breast implants. Plast Reconstr Surg 2004;113:2098-103. [PubMed]
- Peled AW, Duralde E, Foster RD, et al. Patient-reported outcomes and satisfaction after total skin-sparing mastectomy and immediate expander-implant reconstruction. Ann Plast Surg 2014;72 Suppl 1:S48-52. [PubMed]
- Susarla SM, Ganske I, Helliwell L, et al. Comparison of Clinical Outcomes and Patient Satisfaction in Immediate Single-Stage versus Two-Stage Implant-Based Breast Reconstruction. Plast Reconstr Surg 2015;135:1e-8e. [PubMed]
- Strock LL. Two-stage expander implant reconstruction: recent experience. Plast Reconstr Surg 2009;124:1429-36. [PubMed]
- Castelló JR, Garro L, Nájera A, et al. Immediate breast reconstruction in two stages using anatomical tissue expansion. Scand J Plast Reconstr Surg Hand Surg 2000;34:167-71. [PubMed]
- Cordeiro PG, McCarthy CM. A single surgeon’s 12-year experience with tissue expander/implant breast reconstruction: part II. An analysis of long-term complications, aesthetic outcomes, and patient satisfaction. Plast Reconstr Surg 2006;118:832-9. [PubMed]
- Spear SL, Economides JM, Shuck J, et al. Analyzing implant movement with tabbed and nontabbed expanders through the process of two-stage breast reconstruction. Plast Reconstr Surg 2014;133:256e-60e. [PubMed]


