Robotic facelift thyroid surgery
Introduction
Though the traditional Kocher transcervical approach for thyroidectomy has passed the test of time, there has been increasing interest in limiting the cosmetic impact of the resultant large transverse incision at the base of the neck. The use of advanced energy devices such as the harmonic scalpel (Ethicon Endosurgery Inc., Cincinnati, USA) have allowed the development of minimally invasive approaches by which thyroid glands with small nodules could be removed through incisions as small as 1.5 to 2 cm in length. Miccoli is credited with the development of the minimally invasive video assisted thyroidectomy (MIVAT) which utilizes an endoscope, modified retractors, and advanced energy devices to safely perform thyroid surgery through tiny incisions (1). These approaches obviate the need for postoperative drainage and permit safe outpatient surgery in selected patients.
Concurrent with these developments, other surgeons experimented with remote access techniques designed to completely remove any visible incision from the base of the neck. These techniques emerged primarily in Asian centers, where there is a higher risk of hypertrophic scarring and a cultural emphasis on the cosmetic appearance of the anterior neck (2,3). Initial attempts used endoscopes through small incisions placed on the anterior chest, breast, or axilla, or combinations of these sites (4-7). Many of these early remote access techniques for thyroid surgery necessitated CO2 insufflation to maintain the operative space, involved multiple incisions on the breast or chest, and were constrained by 2-dimensional endoscopic visualization and rigid endoscopic instruments. These logistical constraints limited their popularity outside of Asia. The ability to perform thyroid surgery without any visible cervical incision became more feasible with the introduction of the daVinci surgical robot (Intuitive Surgical, Sunnyvale, USA), which allowed the development of a gasless transaxillary approach (8).
As surgeons in the United States began to implement this approach, concerns emerged over the safety of the technique in patients with a larger Western body habitus (9-13). The approach also required placement of drains and necessitated hospital admission, which represented a step backwards from advances made in minimally invasive thyroid surgery during the prior decade. An alternate robotic remote access approach, the robotic facelift thyroidectomy (RFT), was developed to help overcome the concerns and limitations of robotic axillary thyroidectomy in the Western patient population (14,15).
History of procedure
Reports of significant complications not generally associated with thyroid surgery, such as esophageal perforations and brachial plexus injuries emerged with application of the robotic axillary approach into Western practices, so the quest for a better means of eliminating the anterior cervical incision in thyroid surgery continued (9,12,16,17). Experiments evaluating endoscopic access routes to the thyroid compartment in a pig model had indicated that a superiorly-based approach offers improved access to the thyroid, yet required incisions located in cosmetically unfavorable locations (18). Modified facelift approaches had been used in head and neck operations such as parotidectomy and excision of superiorly located neck masses in order to avoid the visible neck incisions associated with more traditional approaches (19,20). These retroauricular approaches were associated with higher patient satisfaction due to improved cosmesis. The concept of retroauricular thyroid surgery combined the improved access of a superiorly based approach with the cosmetic advantages of a modified facelift incision. The key to the feasibility of this approach was the incorporation of the surgical robot, which allowed adequate visualization and intricate dissection through the extended length of the dissection pocket.
Development of the RFT approach began with cadaver dissections in order to assess the feasibility of the procedure (21). After the relevant anatomy was defined, the anticipated surgical approach was performed on seven cadavers. Morphometric analysis of these specimens revealed that the RFT approach required 38% less dissection than the robotic axillary approach.
Terris et al. reported the first use of the RFT approach in patients in 2011, with a series of 14 patients undergoing 18 RFT procedures (22). One patient had a total thyroidectomy through bilateral incisions and three patients had a second contralateral RFT procedure to address malignancies identified at the initial surgery. The mean operative time was 155 minutes, and there were no conversions to an anterior cervical approach. The first patient treated with this technique received a drain and was admitted, but all subsequent procedures were performed without drains in the outpatient setting. There were two seromas and one patient with transient vocal fold weakness in this series, and all of these resolved spontaneously without intervention. All patients reported temporary hypesthesia of the great auricular nerve (GAN) distribution which resolved within several weeks. Permanent vocal fold weakness and hypoparathyroidism did not occur.
Advantages/disadvantages
Remote access thyroidectomy approaches offer the distinct advantage over anterior cervical approaches of completely eliminating a visible neck scar. RFT is our preferred remote access approach for a variety of reasons. The anatomy and vector of dissection is familiar to head and neck surgeons and the brachial plexus is not at risk for injury as it is with the robotic axillary approach (10,12,15). The decreased area of dissection in the RFT approach compared to the axillary approach permits outpatient surgery without drains (14,21). Though the procedure was developed for a unilateral thyroid lobectomy, surgeons have developed robotic techniques to perform total thyroidectomy, bilateral central neck dissection, and an ipsilateral modified neck dissection through a unilateral retroauricular incision (23). While this approach is feasible, it has only been evaluated in a small series of 4 patients. Even in the most experienced hands, this approach took a considerable amount of time and planning, and is still considered experimental.
Despite these advantages, the technique is not without limitations. Transient hypesthesia in the distribution of the GAN is universal, and though it is temporary, patients need to be counseled appropriately because this does not occur through the conventional anterior approach. One main disadvantage of the RFT approach is the increased operative time over a conventional lobectomy (14). Another significant disadvantage in the United States is the additional cost of the procedure. Studies of the robotic transaxillary approach suggest that increased operative time, coupled with the capital expense of the robotic system and the specialized equipment required may significantly increase the cost of thyroid surgery (17,24). While compensation for robotic procedures is significantly higher than conventional approaches in Asia, there is no increased reimbursement in the United States (13).
Patient selection
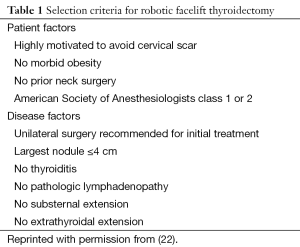
Stringent patient selection criteria for RFT have been created to maximize the likelihood of surgical success while minimizing the risk of complications (Table 1) (22). Candidates for the procedure should be healthy and able to tolerate general anesthesia for several hours. Patients should not be morbidly obese in order to avoid difficulty retracting an excessively thick skin flap, yet patients who are extremely thin are also challenging because elevating a thin skin flap requires very delicate dissection. There should be no prior history of neck surgery, as previous scarring can compromise flap integrity. Diabetes is not a contraindication to the robotic facelift approach, but strict glucose control in the perioperative period is imperative so that risks of wound healing complications are minimized. Finally, the patient must be highly motivated to avoid a visible cervical scar, understands the limitations and risks of the approach, and accept the unlikely chance that conversion to an anterior approach may be required.
Full table
In addition to favorable patient characteristics, the thyroid condition being addressed must also be appropriate for the approach (22). The thyroid disease should be one that is normally treated with unilateral surgery, such as an enlarging or symptomatic benign nodule, a follicular lesion, or follicular lesion of undetermined significance which is being removed for diagnostic purposes. The nodule size should not exceed 4 cm in greatest dimension, and there should be no thyroiditis or history of thyroid compartment surgery in the past. The thyroid lobe should have no substernal extension, and there should be no evidence of a high-grade malignancy such as extrathyroidal extension or pathologic lymphadenopathy.
Patient counseling
Proper preoperative counseling is crucial to patients’ satisfaction of a successful surgical outcome. Patients must understand that RFT carries the standard risks of surgery through the anterior approach, with the added side effect of transient hypesthesia of the region supplied by the GAN. It is also important that they understand that RFT is not minimally invasive surgery, and that the longer operative time, more extensive dissection, and longer recovery period make it more invasive than conventional thyroid surgery. All patients must consent to conversion to an anterior cervical approach in the rare event that it is required. Patients should also understand that, in most instances, the contralateral thyroid lobe cannot be addressed with a unilateral RFT approach. Should the final pathology reveal malignancy, a second RFT on the opposite side or an anterior cervical approach may be required for completion surgery. Finally, patients need to appreciate that this is not a “scarless” procedure; the scar is merely hidden discreetly behind the ear and hairline when it is fully healed rather than at the base of the central neck.
Procedure
The procedure for RFT has been previously described in detail and is summarized in the following text (14,22). A natural neck crease is marked while the patient is sitting upright in the preoperative holding area in the event that an anterior cervical approach is necessary. When the patient is placed on the operating table, he or she should be positioned just off-center of the middle of the table toward the operative side, with the top of the head level with the top of the operating table.
General anesthesia is induced with the aid of a short-acting muscle relaxant, and general anesthesia is maintained with a propofol drip. The patient is subsequently intubated with an electromyographic (EMG) endotracheal tube (ETT) to permit intraoperative laryngeal nerve monitoring. A GlideScope (Verathon Inc, Bothell, USA) is useful during intubation so that all members of the operative team can confirm proper positioning of the EMG electrodes on the ETT. A straight extension is placed on the anesthesia circuit to limit tension and the tubing is then taped to the operating table to prevent inadvertent extubation during the operation. A 3-way stop-cock valve on the end tidal CO2 return tubing prevents kinking of this small caliber tube. The bed is rotated 180°, and the patient’s arms are tucked at their sides and secured with wide silk tape. A formal safety strap that attaches to the table is not used above the patient’s waist because the strap interferes with placement of the retractors on the operating table rails. The patient’s head is turned 20° to 30° away from the operative side, and the head is supported with soft towels.
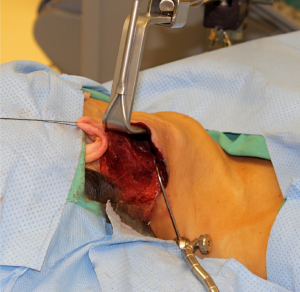
The occipital hairline is shaved one cm posteriorly and the facelift incision is marked behind the hairline so that it will be concealed once the hair re-grows. The incision begins near the inferior extent of the lobule in the postauricular crease, and is carried superiorly and then posteriorly into the shaved region of the occipital hairline in a gentle curve (Figure 1). The incision is carried posteriorly and inferiorly as far as necessary to allow adequate exposure.

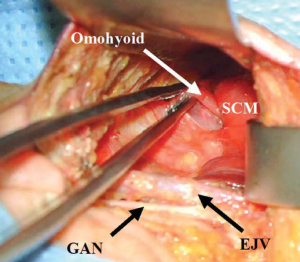
The incision is infiltrated with 0.25% bupivacaine with 1:200,000 epinephrine and the neck is prepped and draped in sterile fashion. The skin is incised with a scalpel and the diathermy is used to develop a subplatysmal flap. The sternocleidomastoid muscle (SCM) is identified, and dissection continues anteriorly and inferiorly along the SCM. The first important structure identified is the GAN. Dissection superficial to the GAN reveals the external jugular vein (EJV) and subsequently the anterior border of the SCM (Figure 2). Ideally, the EJV is preserved, but it may be divided if necessary to improve exposure. The table is placed in reverse Trendelenberg position and rotated away from the surgeon to allow visualization as dissection continues down the anteromedial border of the SCM to the clavicle.
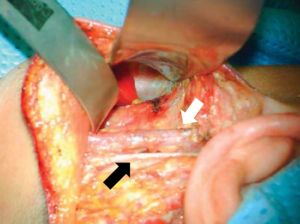
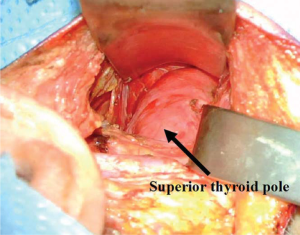
The muscular triangle bordered by the SCM, the omohyoid and the sternohyoid is defined (Figure 3). The omohyoid, sternohyoid and sternothyroid muscles are then retracted ventrally, and the superior pole of the thyroid gland is exposed (Figure 4). The strap muscles are then elevated off of the thyroid lobe, and the superior pedicle is isolated. The modified Chung retractor (Marina Medical, Sunrise, USA) is secured on the contralateral side of the operating table and then positioned so that the strap muscles are retracted ventrally. A Singer hook (Medtronic, Jacksonville FL) attached to a Greenberg retractor (Codman & Shurtleff, Inc., Raynham, USA) secured to the ipsilateral side of the operating table retracts the SCM laterally and dorsally and provides a stable operative field (Figure 5). A modified approach has been described which involves splitting the sternal and clavicular heads of the SCM to provide a similar exposure (25).
The robotic console is positioned on the contralateral side of the patient, with the pedestal angled 30° away from the operating table. Fine adjustments, if necessary, are more easily completed by moving the operating table rather than by moving the robot console. A 30° endoscopic camera is positioned on the robotic arm with the camera facing downward, and then advanced along the long axis of the modified Chung retractor into the surgical field. The camera arm is nearly completely extended in order to avoid collisions with the other two robotic arms. A Harmonic device (Ethicon Endosurgery Inc., Cincinnati, USA) is placed in the dominant robotic arm and a Maryland grasper is placed in the other.
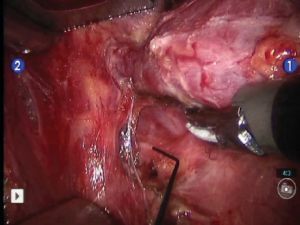
The robotic portion of the procedure begins with the division of the superior pedicle with the Harmonic device. The superior thyroid pole is retracted inferiorly and ventrally to expose the inferior constrictor muscle. This muscle is dissected inferiorly to its lower border, and the superior laryngeal nerve is avoided. The superior parathyroid gland is identified on the posterior aspect of the thyroid and dissected so that the blood supply is preserved. The recurrent laryngeal nerve (RLN) is then identified as it courses under the inferior constrictor (Figure 6). The ligament of Berry is exposed, and then transected with the Harmonic device. The isthmus is divided, and the middle thyroid vein is ligated. The inferior parathyroid gland is identified, and then dissected away from the thyroid gland with its blood supply intact. Lastly, the inferior thyroid vasculature is transected with the Harmonic device, any remaining attachments between the thyroid lobe and the surrounding tissue are lysed and the specimen is removed from the field. The robotic arms are removed and the robotic cart is taken away from the operating table. Surgicel (Ethicon, Inc., Somerville, USA) is placed into the thyroid bed, and the incision is re-approximated using buried interrupted deep dermal 4-0 Vicryl sutures (Ethicon, Inc., Somerville, NJ) without the use of a drain. The skin edges are sealed with Dermaflex tissue adhesive (Chemence Medical Products, Inc., Alpharetta, USA) and 1/4 inch Steri-Strips (3M Corporation, St. Paul, USA) are placed horizontally along the incision. Deep extubation from anesthesia is preferable to minimize coughing or straining which might cause a hematoma.
Results
More than 70 RFT procedures have been performed in our center. In our most recent peer-reviewed publications, 22 RFT procedures in 18 patients were reported (14,22,26). All procedures were completed on an outpatient basis without drainage in all but the first patient of the series. One incidence of transient vocal fold weakness and two seromas occurred, and all resolved without intervention. There have been no episodes of hypocalcemia and no conversions to an anterior cervical approach. Similar experiences have been repeated in at least 6 centers, with more than 300 procedures accomplished with complication profiles similar to those described in our reports.
A direct comparison of RFT and robotic axillary thyroidectomy techniques has been performed (15). The mean operative time for the first ten RFT procedures was 156.9 minutes and was 196 minutes for a group of five axillary approach procedures. Though the case volume was small in this series, the data suggested a shorter learning curve with the RFT approach. All patients treated with robotic axillary thyroidectomy were managed with drains as inpatients, while all but the first RFT patient were discharged on the day of surgery without drains. All patients with the axillary approach experienced chest wall numbness, where those in the RFT group had hypesthesia of the skin in the GAN distribution. No major or permanent complications have been described with the RFT approach to date.
Conclusions
The RFT is a safe and viable alternative to conventional thyroid surgery for selected individuals who wish to avoid a cervical scar. RFT is preferred over robotic axillary thyroidectomy, because it allows outpatient surgery without the need for a drain. As this technique is implemented on a broader scale, strict adherence to patient selection criteria is recommended for optimal outcomes. Training programs for the RFT have not been validated, and this approach should only be performed by experienced high volume endocrine surgeons in specialized centers at the current time. As higher clinical volumes of this procedure reach the literature, a more comprehensive understanding of the potential and limitations of this procedure will become available.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Miccoli P, Berti P, Conte M, et al. Minimally invasive surgery for thyroid small nodules: preliminary report. J Endocrinol Invest 1999;22:849-51. [PubMed]
- McCurdy JA Jr. Considerations in Asian cosmetic surgery. Facial Plast Surg Clin North Am 2007;15:387-97. vii. [PubMed]
- Duh QY. Robot-assisted endoscopic thyroidectomy: has the time come to abandon neck incisions? Ann Surg 2011;253:1067-8. [PubMed]
- Ohgami M, Ishii S, Arisawa Y, et al. Scarless endoscopic thyroidectomy: breast approach for better cosmesis. Surg Laparosc Endosc Percutan Tech 2000;10:1-4. [PubMed]
- Ikeda Y, Takami H, Niimi M, et al. Endoscopic thyroidectomy by the axillary approach. Surg Endosc 2001;15:1362-4. [PubMed]
- Shimazu K, Shiba E, Tamaki Y, et al. Endoscopic thyroid surgery through the axillo-bilateral-breast approach. Surg Laparosc Endosc Percutan Tech 2003;13:196-201. [PubMed]
- Choe JH, Kim SW, Chung KW, et al. Endoscopic thyroidectomy using a new bilateral axillo-breast approach. World J Surgr;31:601-6.
- Kang SW, Lee SC, Lee SH, et al. Robotic thyroid surgery using a gasless, transaxillary approach and the da Vinci S system: the operative outcomes of 338 consecutive patients. Surgery 2009;146:1048-55. [PubMed]
- Kandil EH, Noureldine SI, Yao L, et al. Robotic transaxillary thyroidectomy: an examination of the first one hundred cases. J Am Coll Surg 2012;214:558-64. [PubMed]
- Landry CS, Grubbs EG, Warneke CL, et al. Robot-assisted transaxillary thyroid surgery in the United States: is it comparable to open thyroid lobectomy? Ann Surg Oncol 2012;19:1269-74. [PubMed]
- Perrier ND. Why I have abandoned robot-assisted transaxillary thyroid surgery. Surgery 2012;152:1025-6. [PubMed]
- Kuppersmith RB, Holsinger FC. Robotic thyroid surgery: an initial experience with North American patients. Laryngoscope 2011;121:521-6. [PubMed]
- Dionigi G. Robotic thyroidectomy: Seoul is not Varese. Otolaryngol Head Neck Surg 2013;148:178. [PubMed]
- Terris DJ, Singer MC, Seybt MW. Robotic facelift thyroidectomy: II. Clinical feasibility and safety. Laryngoscope 2011;121:1636-41. [PubMed]
- Terris DJ, Singer MC. Qualitative and quantitative differences between 2 robotic thyroidectomy techniques. Otolaryngol Head Neck Surg 2012;147:20-5. [PubMed]
- Perrier ND, Randolph GW, Inabnet WB, et al. Robotic thyroidectomy: a framework for new technology assessment and safe implementation. Thyroid 2010;20:1327-32. [PubMed]
- Inabnet WB 3rd. Robotic thyroidectomy: must we drive a luxury sedan to arrive at our destination safely? Thyroid 2012;22:988-90. [PubMed]
- Terris DJ, Haus BM, Nettar K, et al. Prospective evaluation of endoscopic approaches to the thyroid compartment. Laryngoscope 2004;114:1377-82. [PubMed]
- Terris DJ, Tuffo KM, Fee WE Jr. Modified facelift incision for parotidectomy. J Laryngol Otol 1994;108:574-8. [PubMed]
- Roh JL. Retroauricular hairline incision for removal of upper neck masses. Laryngoscope 2005;115:2161-6. [PubMed]
- Singer MC, Seybt MW, Terris DJ. Robotic facelift thyroidectomy: I. Preclinical simulation and morphometric assessment. Laryngoscope 2011;121:1631-5. [PubMed]
- Terris DJ, Singer MC, Seybt MW. Robotic facelift thyroidectomy: patient selection and technical considerations. Surg Laparosc Endosc Percutan Tech 2011;21:237-42. [PubMed]
- Byeon HK, Holsinger FC, Tufano RP, et al. Robotic total thyroidectomy with modified radical neck dissection via unilateral retroauricular approach. Ann Surg Oncol 2014;21:3872-5. [PubMed]
- Cabot JC, Lee CR, Brunaud L, et al. Robotic and endoscopic transaxillary thyroidectomies may be cost prohibitive when compared to standard cervical thyroidectomy: a cost analysis. Surgery 2012;152:1016-24. [PubMed]
- Saeed A, Alsaleh N, Moulthrop T, et al. Modified Approach for Robotic Retroauricular Thyroidectomy: Preclinical Simulation and a Surgical Case. Surg Innov 2014. [Epub ahead of print]. [PubMed]
- Terris DJ, Singer MC. Robotic facelift thyroidectomy: Facilitating remote access surgery. Head Neck 2012;34:746-7. [PubMed]