Approach to the surgical management of primary aldosteronism
Introduction
Primary aldosteronism (PA) is an adrenal disease which causes sodium and water retention and potassium excretion, with ensuing hypertension and hypokalemia.
This condition was first described by Litynski in Poland and Conn in the USA (1,2). Recent guidelines have defined PA as “a group of disorders in which aldosterone production is inappropriately high, relatively autonomous from the renin-angiotensin system, and non-suppressible by sodium loading” (3).
The guidelines recommend case detection of PA by using the aldosterone-renin ratio (ARR) measured under standard conditions, followed by one of four commonly used confirmatory tests (oral sodium loading, saline infusion, fludrocortisone suppression, and captopril challenge) (3), although the accuracy of these tests has been called into question (4).
Despite being regarded as a very rare (less than 1%) cause of arterial hypertension for several decades, PA has been now recognized to be the most common cause of endocrine hypertension, since it was found to involve more than 11% of referred hypertensive patients (5-8).
Because of its prevalence, and also because patients with PA have higher cardiovascular morbidity and mortality than age-, blood pressure (BP)- and sex-matched patients with essential hypertension (9,10), it is important to search for PA in a systematic way at least in some categories of patients. These include patients with Joint National Commission stage 2 (>160-179/100-109 mmHg), stage 3 (>180/110 mmHg), or drug-resistant hypertension; hypertension and spontaneous or diuretic-induced hypokalemia; hypertension with adrenal incidentaloma; or hypertension and a family history of early-onset hypertension or cerebrovascular accident at a young age (<40 years). Case detection is also recommended for all hypertensive first-degree relatives of patients with PA (3) (Table 1).

Full table
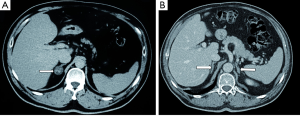
PA may be caused by unilateral adrenal involvement [aldosterone producing adenoma (APA)] (Figure 1A), which is considered a surgically curable form, and bilateral adrenal involvement (idiopathic adrenal hyperplasia) that is considered non-surgically curable. APA and idiopathic adrenal hyperplasia were claimed to account for more than 95% of all cases (11), but several studies have recently reported novel variants of surgically treatable PA, including diffuse or nodular unilateral adrenal hyperplasia (UAH) (Figure 1B). This variant was previously considered to be a very rare disease (0.1%) (11), but was thereafter found to involve from 8% to 70% of cases of surgically treated PA (12-16).

The normalization of aldosterone is a fundamental goal of treatment, because chronical hyperaldosteronism concurs with high BP in causing cardiovascular complications, including, left ventricular hypertrophy, arterial wall stiffening, metabolic syndrome, renal damage, myocardial infarction and atrial fibrillation (17,18).
The role of lateralizing techniques
Only patients with lateralized adrenal hypersecretion can be cured by unilateral adrenalectomy; hence, the distinction between unilateral and bilateral aldosterone hypersecretion is the key.
To this end adrenal venous sampling (AVS), computerized tomography (CT), magnetic resonance imaging (MRI) and adrenocortical scintigraphy have been used.
AVS was introduced in the late 1960s and became the first test to distinguish unilateral from bilateral PA (19). Adrenocortical scintigraphy, CT and MRI were developed later and were adopted as primary procedures to differentiate unilateral from bilateral adrenal abnormalities, because they are less invasive than AVS.
Adrenal venous sampling
AVS involves the measurement of aldosterone and cortisol levels in the infra-adrenal inferior vena cava and in the adrenal veins of both sides (Figure 2). Considering the variability of aldosterone secretion, some experts advocate AVS during exogenous adrenocorticotropic hormone (ACTH) infusion to maximize cortisol secretion, enhancing the assessment of selectivity of catheterization and to maximize differences of aldosterone between sides (13). Other experts suggest AVS in the early morning, when ACTH secretion peaks (3). Most experts agree that ACTH does facilitate the assessment of selectivity, but may confound the ascertainment of lateralization; to the contrary, a comparative study reported that exogenous ACTH infusion does not improve the detection of unilateral aldosterone hypersecretion if the two adrenal veins are catheterized simultaneously (20).
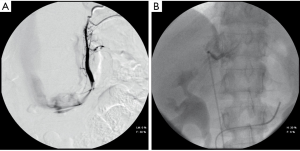
There is not general agreement regarding the definition of successful selectivity and lateralization in AVS (3). Catheter insertion is considered successful under unstimulated conditions, if cortisol concentrations are 1.1 to 3 times higher in the adrenal veins than in the inferior vena cava or in a peripheral vein. Aldosterone secretion is considered to be lateralized if the aldosterone to cortisol ratio is 2 to 5 times higher on the dominant side than contra-laterally.
AVS has a rate of failure between 3% and 22% (21,22). It is an invasive procedure, which carries a very tiny risk of complications, but requires skilled radiologist to minimize morbidity (23,24). The only clinically relevant complication is the rupture of the adrenal veins (0.6%) which is usually managed with conservative treatment (23).
The key message is that the objective of surgery is to resolve the aldosterone hypersecretion, and not just to remove a nodule. Since aldosterone hypersecretion can occur even with no adrenal enlargement (as detected by CT and MRI), AVS was found to be superior to image-based techniques for surgical decisions, as unilateral hypersecretion may be associated with UAH that can be undetectable on usual imaging techniques (13,22,25).
Recently, some authors tried to develop a score to avoid the use of AVS. Küpers et al. published data on a clinical prediction score (CPS), developed to diagnose unilateral PA, using a combination of biochemical and radiological characteristics (26). By applying this score, they reported that 30% of the cohort of patients could potentially have avoided AVS and proceeded straight to surgery. Likewise Sze et al. used CPS for a prospectively gathered cohort of patients with PA (27). The results suggest that CPS has the potential to lead to occasional erroneous decision-making regarding patient selection for adrenalectomy and therefore CPS may be useful for clinical decisions only in cases of unsuccessful AVS or in centers where AVS is not available.
Iodo-cholesterol scintigraphy
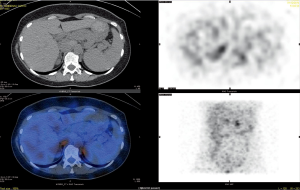
[131I] 19-Iodocholesterol scintigraphy was first used in the early 1970s (28). The NP-59 scan [6-131I] iodomethyl-19-norcholesterol (NP-59), performed with dexamethasone suppression, was introduced in 1977 and had the advantage of correlating anatomical abnormalities with function (29). However, its sensitivity depends heavily on the size and the degree of hyperfunction of the adenoma (30,31). Improved imaging resolution achieved by the introduction of the multiple head gamma camera and Single Photon Emission Computed Tomography (SPECT) technique enhanced the ability of NP-59 scintigraphy to detect smaller adrenal lesions (32) (Figure 3).
However, because of a poor tracer uptake in adenomas smaller than 1.5 cm in diameter, this technique is often not helpful in interpreting micronodular findings obtained with high-resolution CT (33).
In 2009 a study by Yen et al. evaluated the ability of NP-59 scintigraphy to predict the outcome in PA after adrenalectomy (34). The postsurgical outcomes were consistent with the presurgical NP-59 SPECT/CT results: 16 out of 23 patients showed unilateral NP-59 uptake, and 10 of these 16 (62.5%) showed postsurgical improvement; of seven patients with negative NP-59 SPECT/CT results, none showed postsurgical clinical improvement.
In 2014 Lu et al. applied a similar approach in a study of 49 patients, of which 40 had an APA and 9 had hyperplasia. They concluded that semiquantification of NP-59 scintigraphy shows an ability similar to AVS in differentiating APA from bilateral idiopathic adrenal hyperplasia and has an excellent ability to predict postsurgical outcomes of adrenalectomy (35).
However, other series have found misleading results in establishing lateralization with NP-59 (36). Moreover, NP-59 is an expensive and time-consuming procedure and its use is limited by the poor availability of the radiotracer (37).
Image-based techniques
CT is the most widely used imaging test (Figure 4); although less diffuse, MRI performs similarly (38).
Most aldosterone-producing adenomas are less than 20 mm in diameter (25,39). The presence of a small adrenal nodule in patients with PA suggests the presence of an APA, but it does not exclude the combination of a non-secreting adenoma and idiopathic PA (3,40).
A meta-analysis including 38 studies (6) demonstrated that the proportion of patients with unilateral nodule on CT or MRI imaging, and a bilateral or contralateral secretion documented by AVS, was about 20%.
Since the prevalence of non-secreting adenomas increase with age, their presence is less likely in young patients with PA. Thus, the occurrence of an isolated characteristic adrenal nodule in PA patients aged less than 40 has been proposed as a surrogate for diagnosing unilateral aldosterone hypersecretion (8,13), although results from a recent series do not support this strategy (36).
The guidelines recommend CT in all patients with confirmed PA to detect an adrenal carcinoma, even when an adrenalectomy is not considered (3). However, adrenal carcinomas presenting as isolated PA are rare (2.5% of patients with adrenocortical carcinoma, which represents 0.05-0.2% of all malignancies) (41).
Moreover, CT and MRI results were found to disagree with AVS in a considerable proportion of patients. CT/MRI would show, for example, a unilateral abnormal adrenal gland when aldosterone hypersecretion was occurring in the contralateral gland or bilaterally, or would show bilateral normal or abnormal adrenal glands when aldosterone hypersecretion was occurring in only one gland (22,42-45).
A prospective study of 203 patients showed that operative planning based on anatomical imaging alone would have inappropriately excluded 21.7% of patients from adrenalectomy and would have led to unnecessary surgery in 24.7%, which led to a “renaissance” of AVS (13).
A recent systematic review concluded that when AVS is used as the standard criterion test for the lateralization of aldosterone hypersecretion in patients with PA, CT and MRI may misdiagnose the cause of PA in 37.8% of patients (6).
Thus, most experts now agree that the referral diagnostic test for lateralization of aldosterone hypersecretion is AVS, because the interpretation of other imaging techniques (CT, MRI, adrenocortical scintigraphy) may lead to inappropriate treatment of PA patients (3,6,12,13).
Medical treatment vs. surgery
In APA and other unilateral PA variants, adrenalectomy represents the elective treatment, while the administration of mineralocorticoid receptor antagonists (MRAs) is indicated for patients with bilateral disease and non-surgically correctable PA. The two main MRAs currently available are spironolactone and eplerenone (46); the guidelines recommend spironolactone as the primary agent for the management of PA, with eplerenone as an alternative (3). Potassium canrenoate and/or canrenone may be also used.
MRAs or potassium chloride are commonly prescribed also to hypokalemic patients with APAs to correct hypokalemia before surgery, in order to avoid the risk of hypokalemia-related arrhythmia during anesthesia (47). The preoperative treatment does not increase the incidence of hypoaldosteronism or hyperkalemia after surgery (48).
Some studies that compared spironolactone to surgery also in patients with APA reported no differences between treatments for the control of either BP or serum potassium concentrations (49). Some studies with long-term follow up seem to suggest that spironolactone may induce a regression of left ventricular hypertrophy (50).
In the few studies on echocardiographic follow-up of PA patients treated by either adrenalectomy or MRAs, adrenalectomy was almost systematically found to rapidly decrease left ventricular mass (LVM), while the effects of MRAs were more controversial (51-54). According to the meta-analysis of five selected studies, there would be no significant difference in LVM change between patients with PA undergoing adrenalectomy or medical treatment with MRAs, despite a greater effect of surgery in reducing BP (55).
However, the impact on the quality of life and costs should be also considered.
In fact, spironolactone has adverse effects such as gynaecomastia, mastodynia, menstrual abnormalities and erectile disfunction (56); otherwise the newest eplerenone is better tolerated, but more expensive and less effective (57).
A recent study reported that the quality of life would be lower in patients with unilateral PA than in the general population, while administration of spironolactone or amiloride would improve quality of life scores after 6 months. However, the improvement was slower and smaller in medically treated than in operated patients (58).
According to some authors, it remains uncertain whether adrenalectomy for lateralized disease should routinely be offered to PA patients (59). Ideally, a prospective randomized clinical trial could be performed, but it could be extremely challenging to manage, given the large number of patients and the length of the follow-up required to demonstrate a meaningful difference in cardiovascular outcomes.
Reimel et al. attempted to compare the cost-effectiveness of a guideline-based surgical strategy with universal pharmacologic management, using a Markov state transition model (60). They demonstrated that screening for PA and resecting an APA was the least costly strategy. Even though most APA patients can be managed medically, in order to make universal pharmacologic therapy the less costly strategy for patients with an approximate 40-year life expectancy, the probability of finding a unilateral APA should be less than 10%; the cost of adrenalectomy should be more than double; the cost of AVS should be more than 4-fold higher than current and the surgical failure rate should be more than sextuple.
Surgical procedures
Open vs. laparoscopic adrenalectomy
Open removal of the aldosterone secreting tumors, by either the transabdominal or the lumbar approach, was unchallenged until 1992, when Gagner, Suzuki and Higashihara published the first series of laparoscopic excision, performed via a transperitoneal approach (61-63). The supine anterior approach was described in 1995 (64), whilst other authors have described the retroperitoneoscopic approach (65,66).
Laparoscopic adrenalectomy (LA), has now been proven to be a safe and effective treatment for PA (67); laparoscopically treated patients have fewer postoperative complications and are equally likely to improve in BP and correct hypokalemia, if present preoperatively, when compared with patients treated with open adrenalectomy. Additional benefits of LA are smaller incisions, decreased postoperative pain, and a shorter hospital stay (68,69).
In a review by Steichen et al., LA showed a morbidity of 5-14%, a mortality below 1%, and a mean hospital stay around 3 days (70). The increasing confidence in this technique led to proposal for adrenalectomy also in an outpatient setting (71).
Complications of laparoscopic surgery may include conversion to open surgery, hematoma due to intraoperative vascular injury, thromboembolism, pneumothorax or hemothorax.
Adrenalectomy generally results in the normalization of aldosterone secretion and in close to 100% of the cases if patients are selected with AVS, and in a large decrease of BP and antihypertensive medication; however normotension without treatment is achieved only in 42% of cases (70).
Transperitoneal vs. retroperitoneal approach
Laparoscopic surgery using the transperitoneal or retroperitoneal approaches is currently the preferred strategy to treat patients with unilateral PA (72,73) (Figures 5,6).
In general, three recent meta-analyses compared the transperitoneal and retroperitoneal adrenalectomy: two concluded that both techniques have equivalent outcomes (74,75) whereas the other claimed the retroperitoneal approach to be superior in short-term outcomes (76).
In the transperitoneal approach, the procedure is performed with the patient in lateral decubitus flank position. Pneumoperitoneum (at 12-14 mmHg by CO2) is usually made by Hasson cannula inserted by open technique. A subcostal port is placed for the laparoscope, and two/three other 5/10 mm ports sited.
For right adrenalectomy the liver is mobilized and retracted during the procedure. The renal vein represents the inferior margin of the dissection and the right main adrenal vein is identified by following the lateral edge of the vena cava. The dissection displays the inferior vena cava and nearby the adrenal gland (Figure 7). The main right adrenal vein is identified and divided early. Gland dissection is continued superiorly; the vascular branches from the inferior phrenic vessels, the aorta, and the renal vessels are divided.
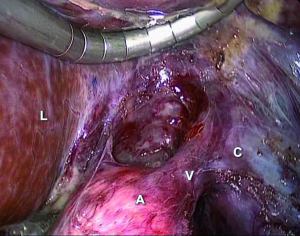
On the left side the colonic flexure is mobilized, the splenorenal ligament is dissected to allow the spleen to fall away. As spleen fall medially, the tail of the pancreas and the splenic vein and artery are identified. The peritoneum along the inferior border of the pancreas is divided: an avascular plane separating the adrenal gland from the tail of the pancreas and the splenic vein is opened. The dissection and the traction of the splenic vein allow viewing the inferior adrenal vein, going into the left renal vein (Figure 8). The inferior adrenal vein is divided and then the gland is dissected and removed. The inferior phrenic vein runs close to the left adrenal gland and empties into the adrenal vein: it may be preserved or divided. Small arterial vessel from superior, medium and inferior pedicle can be easily coagulated. In all cases, the adrenal gland is removed via a retrieval bag.
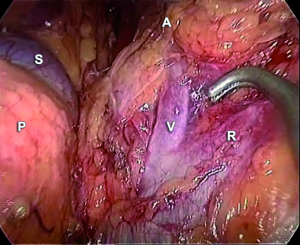
In the retroperitoneoscopic approach, the procedure is performed with the patient in the prone position. A 1.5 cm transverse incision just below the tip of the 12th rib is performed. A small cavity is prepared digitally for insertion under finger guidance of one 5 mm trocar 4 to 5 cm laterally. In the same way, a 10 mm trocar is inserted 4 to 5 cm medial to the initial wound. A blunt trocar with an inflatable balloon is introduced into the initial incision and the capnoretroperitoneum is created (at 20-22 mmHg). Following creation of the retroperitoneal space by pushing down the fatty tissue, the upper pole of the kidney is retracted and the area of the adrenal gland exposed.
On the right side, lifting up the adrenal gland, the inferior vena cava is visualized posteriorly; the short right main adrenal vein running postero-laterally becomes visible; thus, it is divided. The preparation of the right adrenal gland is completed by lateral and cranial dissection. For left-sided retroperitoneoscopic adrenalectomy, the adrenal gland vein must be prepared in the space between the adrenal gland and the diaphragmatic branch, medial to the upper pole of the kidney (Figure 9). After dissection of the inferior main vein, the preparation of the adrenal gland is continued medially, laterally, and cranially by lifting the gland at its venous stump. In all cases, the fully mobilized adrenal gland is placed in a retrieval bag and pulled through the initial incision.
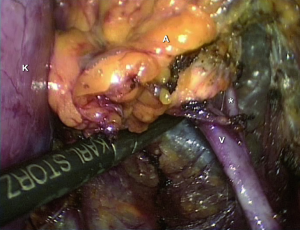
Total vs. partial “sparing”adrenalectomy
For a long time, unilateral total adrenal excision has been considered the technique of choice in patients with surgically correctable PA. However, the routine unilateral partial adrenalectomy has also been advocated in order to preserve the remnant adrenal function and avoid potential adrenal insufficiency (77).
This strategy may expose the patient to increased risk of failures because of incomplete excision with subsequent persistent and recurrent PA. Furthermore, Honda et al. demonstrated that more than 80% of the adrenal reserve capacity is preserved also after unilateral total adrenalectomy in patients with APA without subclinical or overt Cushing’s syndrome, making the risk of corticosteroid replacement after unilateral total adrenalectomy unlikely (78).
Hence, the indication to laparoscopic total or partial adrenalectomy in patients with unilateral PA remains controversial; the risk-to-benefit ratio must be weighed against the potential advantage of partial adrenalectomy.
Walz suggested that APA could be an ideal indication for partial adrenalectomy, because these tumors are almost always benign, small, and often lie eccentrically (79). An endoscopic ultrasound probe has been considered extremely useful for identifying the margin of normal and tumor tissue (73). Some studies suggest that partial adrenalectomy may achieve the biochemical cure of disease in almost all patients.
Nakada and Al-Sobhi reported the results of 26 and 7 adrenal sparing procedures at a mean follow up of 5 years and 1 year, respectively, without any recurrent hyperaldosteronism and with a greater reserve of normal ipsilateral adrenal tissue (80,81).
Jeschke et al. reported a series of laparoscopic partial adrenalectomy in 13 patients with APA with a low complication rate along with unremarkable postoperative BP and aldosterone levels (82).
In 2011, Fu et al. published the results of a multicenter randomized study comparing retroperitoneoscopic partial to total adrenalectomy for APA in 212 patients, with a mean follow up of 96 months in each group (77). No significant difference in age, gender, adrenalectomy site or tumor size between the two groups were found, although AVS was performed only in patients with equivocal cross-sectional imaging findings (contralateral adrenal gland enlargement or an adrenal neoplasm smaller than 1 cm). Patients undergoing partial adrenalectomy (n=104) had short and long-term results similar to those who underwent total adrenal excision (n=108). However, they had a greater blood loss that did not require blood transfusions; the authors concluded that retroperitoneoscopic partial adrenalectomy is technically safe and effective in patients with PA due to APA.
By contrast, other authors have reported some failures following partial adrenalectomy. In 2003, Fendrich et al. reported a persistent hyperaldosteronism following incomplete adrenalectomy (83). In this case, CT clearly visualized two tumors in one adrenal gland; in the first operation, the nonfunctioning lesion was removed and a second operation was necessary to achieve the cure. In 2005, Ishidoya et al. performed a comparative, nonrandomized study of surgical outcomes in two groups of patients with APA who underwent laparoscopic partial and total adrenalectomy (84). The authors performed 29 partial adrenalectomies and experienced two cases in which hypertension and hyperaldosteronism failed to improve. Preoperative AVS and high resolution CT identified a unilateral single APA; thus, it was concluded that another functioning microadenoma was missed in the ipsilateral adrenal gland.
Hennings et al. reported two recurrences in nodular UAH after subtotal adrenalectomy; in one of these patients, relapse was caused by recurrent ipsilateral nodular disease in the remnant (16).
These failures may occur because APA cannot be predicted accurately before surgery, and UAH may be more common than expected (85). Subsequently, partial adrenalectomy in cases of unsuspected UAH may theoretically achieve an incomplete removal of abnormal adrenal tissue.
In fact, Shigematsu et al. found that aldosterone hypersecretion is not localized exclusively in the adenoma cells but also in the adjacent hyperplastic or micronodular tissue (86). However, little is known about how the cells expressing the enzyme for aldosterone synthase are distributed in the adrenal glands; new findings in immunohistochemistry and immunofluorescence may provide useful tools for diagnosis and treatment of adrenocortical adenomas (87,88).
KCNJ5 and surgery
Recently, important insights of the underlying cause of APA were presented. In 2011 Choi et al. identified three mutations in the selectivity filter of the KCNJ5 (Kir 3.4) potassium channel (89). These mutations result in loss of channel selectivity followed by entry of Na+, chronic depolarization, constitutive aldosterone production, and cell proliferation.
In a recent paper, Dekkers et al. underline that most adrenal glands with supposedly unilateral aldosterone production display multinodular pathology (90). Somatic mutations in KCNJ5 and other genes (ATP1A1 or CACNA1D) are not limited to APAs but are also found in the more frequent multinodular adrenals. However, in multinodular glands, only one nodule (usually the largest) harbors a mutation. This suggests that the occurrence of a mutation and nodule formation are independent processes. However, the relevance of these findings for clinical management remains to be defined.
In a recent study including 28 patients, Arnesen et al. found that 36% of patients with PA displayed tumor mutations in KCNJ5 (91). The presence of KCNJ5 mutations was associated with lower BP and a higher chance for cure by surgery when compared to patients harboring the KCNJ5 wild type. Thus, a preoperative identification of the mutation status might have impact on surgical strategy.
Rossi et al. described that patients with APA carrying somatic mutations in the KCNJ5 showed more prominent cardiovascular damage than wild-type APA patients (92), presumably reflecting long-term exposure to higher aldosterone levels (93). However, the chances of these patients being cured from the hyperaldosteronism and high BP, and regression of left ventricular hypertrophy after adrenalectomy, were not compromised by the presence of these mutations.
KCNJ5 mutations are common among Japanese APA patients (69.4%) (94). Kitamoto et al. found that the KCNJ5-mutated patients demonstrated significant postoperative improvements in LVM index compared to patients harboring the KCNJ5 wild type, possibly due to strong autonomous aldosterone production (94).
Although not definitive, all these findings may have relevant implications for the management of PA.
Outcome of surgery
The goal of surgery in PA should be the cure of aldosterone excess because of its deleterious direct effect on the cardiovascular system (17,18). Interestingly, in several papers the outcomes of surgery focus only on BP changes and the normalization of serum potassium levels is often used as a surrogate of PA recovery.
When unilateral disease has been unequivocally established, adrenalectomy achieves the correction of hypokalemia, if present before surgery, in virtually all patients (36); cure of hypertension is reported in about 30-60% of cases, and in the remaining patients a marked improvement of BP values is described (52,70,95).
The wide variation of BP outcome after adrenalectomy could be explained by the different criteria used to define cure and improvement, and even more by the fact that BP is a highly complex phenotype, deriving from several factors. For example, a patient with no cure of PA after adrenalectomy can become normotensive just for lifestyle measures and/or because of myocardial infarction. Conversely, a patient with concomitant essential hypertension is unlikely to be definitively cured even if the PA is fully corrected. Hence, we strongly advocate the need of considering the postoperative normalization of ARR as the main endpoint for determining outcomes of PA.
Several authors have suggested potential predictors of a postoperative hypertension cure in PA patients, including female sex, young age, no family history of hypertension, short duration of hypertension, low preoperative BP, few prescribed antihypertensive drug classes, low body mass index, and high estimated glomerular filtration rate (25,70,93,95-98).
Zarnegar et al. developed the Aldosteronoma Resolution Score, which was composed of four predictors (number of antihypertensive drugs, body mass index, duration of hypertension, sex) and roughly estimated postoperative hypertension cure according to 3 categories of likelihood (97).
Utsumi et al. have recently proposed a more accurate nomogram that could predict hypertension cure in PA patients after LA. Age, sex duration of hypertension, and number of antihypertensive drug classes are included in the nomogram (98). These scores/nomograms can help clinicians to calculate the probability of postoperative hypertension cure in PA patients and inform patients preoperatively.
Acknowledgements
The authors wish to thank Mrs Ruth Phillips for help in English revision.
Disclosure: The authors declare no conflict of interest.
References
- Kucharz EJ. Michał Lityński--a forgotten author of the first description on primary hyperaldosteronism. Pol Arch Med Wewn 2007;117:57-8. [PubMed]
- CONN JW. Presidential address. I. Painting background. II. Primary aldosteronism, a new clinical syndrome. J Lab Clin Med 1955;45:3-17. [PubMed]
- Funder JW, Carey RM, Fardella C, et al. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2008;93:3266-81. [PubMed]
- Rossi GP, Seccia TM, Pessina AC. A diagnostic algorithm--the holy grail of primary aldosteronism. Nat Rev Endocrinol 2011;7:697-9. [PubMed]
- Rossi GP. Prevalence and diagnosis of primary aldosteronism. Curr Hypertens Rep 2010;12:342-8. [PubMed]
- Kempers MJ, Lenders JW, van Outheusden L, et al. Systematic review: diagnostic procedures to differentiate unilateral from bilateral adrenal abnormality in primary aldosteronism. Ann Intern Med 2009;151:329-37. [PubMed]
- Rossi GP, Bernini G, Caliumi C, et al. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol 2006;48:2293-300. [PubMed]
- Mulatero P, Stowasser M, Loh KC, et al. Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J Clin Endocrinol Metab 2004;89:1045-50. [PubMed]
- Milliez P, Girerd X, Plouin PF, et al. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol 2005;45:1243-8. [PubMed]
- Stowasser M, Sharman J, Leano R, et al. Evidence for abnormal left ventricular structure and function in normotensive individuals with familial hyperaldosteronism type I. J Clin Endocrinol Metab 2005;90:5070-6. [PubMed]
- Goh BK, Tan YH, Chang KT, et al. Primary hyperaldosteronism secondary to unilateral adrenal hyperplasia: an unusual cause of surgically correctable hypertension. A review of 30 cases. World J Surg 2007;31:72-9. [PubMed]
- Trésallet C, Salepçioglu H, Godiris-Petit G, et al. Clinical outcome after laparoscopic adrenalectomy for primary hyperaldosteronism: the role of pathology. Surgery 2010;148:129-34. [PubMed]
- Young WF, Stanson AW, Thompson GB, et al. Role for adrenal venous sampling in primary aldosteronism. Surgery 2004;136:1227-35. [PubMed]
- Quillo AR, Grant CS, Thompson GB, et al. Primary aldosteronism: results of adrenalectomy for nonsingle adenoma. J Am Coll Surg 2011;213:106-12. [PubMed]
- Novitsky YW, Kercher KW, Rosen MJ, et al. Clinical outcomes of laparoscopic adrenalectomy for lateralizing nodular hyperplasia. Surgery 2005;138:1009-16. [PubMed]
- Hennings J, Andreasson S, Botling J, et al. Long-term effects of surgical correction of adrenal hyperplasia and adenoma causing primary aldosteronism. Langenbecks Arch Surg 2010;395:133-7. [PubMed]
- Strauch B, Petrák O, Zelinka T, et al. Adrenalectomy improves arterial stiffness in primary aldosteronism. Am J Hypertens 2008;21:1086-92. [PubMed]
- Rossi GP, Bernini G, Desideri G, et al. Renal damage in primary aldosteronism: results of the PAPY Study. Hypertension 2006;48:232-8. [PubMed]
- Melby JC, Spark RF, Dale SL, et al. Diagnosis and localization of aldosterone-producing adenomas by adrenal-vein cateterization. N Engl J Med 1967;277:1050-6. [PubMed]
- Rossi GP, Ganzaroli C, Miotto D, et al. Dynamic testing with high-dose adrenocorticotrophic hormone does not improve lateralization of aldosterone oversecretion in primary aldosteronism patients. J Hypertens 2006;24:371-9. [PubMed]
- Daunt N. Adrenal vein sampling: how to make it quick, easy, and successful. Radiographics 2005;25 Suppl 1:S143-58. [PubMed]
- Magill SB, Raff H, Shaker JL, et al. Comparison of adrenal vein sampling and computed tomography in the differentiation of primary aldosteronism. J Clin Endocrinol Metab 2001;86:1066-71. [PubMed]
- Rossi GP, Barisa M, Allolio B, et al. The Adrenal Vein Sampling International Study (AVIS) for identifying the major subtypes of primary aldosteronism. J Clin Endocrinol Metab 2012;97:1606-14. [PubMed]
- Young WF, Stanson AW. What are the keys to successful adrenal venous sampling (AVS) in patients with primary aldosteronism? Clin Endocrinol (Oxf) 2009;70:14-7. [PubMed]
- Letavernier E, Peyrard S, Amar L, et al. Blood pressure outcome of adrenalectomy in patients with primary hyperaldosteronism with or without unilateral adenoma. J Hypertens 2008;26:1816-23. [PubMed]
- Küpers EM, Amar L, Raynaud A, et al. A clinical prediction score to diagnose unilateral primary aldosteronism. J Clin Endocrinol Metab 2012;97:3530-7. [PubMed]
- Sze WC, Soh LM, Lau JH, et al. Diagnosing unilateral primary aldosteronism - comparison of a clinical prediction score, computed tomography and adrenal venous sampling. Clin Endocrinol (Oxf) 2014;81:25-30. [PubMed]
- Conn JW, Morita R, Cohen EL, et al. Primary aldosteronism. Photoscanning of tumors after administration of 131 I-19-iodocholesterol. Arch Intern Med 1972;129:417-25. [PubMed]
- Sarkar SD, Cohen EL, Beierwaltes WH, et al. A new and superior adrenal imaging agent, 131I-6beta-iodomethyl-19-nor-cholesterol (NP-59): evaluation in humans. J Clin Endocrinol Metab 1977;45:353-62. [PubMed]
- Hogan MJ, McRae J, Schambelan M, et al. Location of aldosterone-producing adenomas with 131I-19-iodocholesterol. N Engl J Med 1976;294:410-4. [PubMed]
- Nomura K, Kusakabe K, Maki M, et al. Iodomethylnorcholesterol uptake in an aldosteronoma shown by dexamethasone-suppression scintigraphy: relationship to adenoma size and functional activity. J Clin Endocrinol Metab 1990;71:825-30. [PubMed]
- Hwang I, Balingit AG, Georgitis WJ, et al. Adrenocortical SPECT using iodine-131 NP-59. J Nucl Med 1998;39:1460-3. [PubMed]
- Mansoor GA, Malchoff CD, Arici MH, et al. Unilateral adrenal hyperplasia causing primary aldosteronism: limitations of I-131 norcholesterol scanning. Am J Hypertens 2002;15:459-64. [PubMed]
- Yen RF, Wu VC, Liu KL, et al. 131I-6beta-iodomethyl-19-norcholesterol SPECT/CT for primary aldosteronism patients with inconclusive adrenal venous sampling and CT results. J Nucl Med 2009;50:1631-7. [PubMed]
- Lu CC, Wu VC, Wu KD, et al. Prognostic value of semiquantification NP-59 SPECT/CT in primary aldosteronism patients after adrenalectomy. Eur J Nucl Med Mol Imaging 2014;41:1375-84. [PubMed]
- Citton M, Viel G, Rossi GP, et al. Outcome of surgical treatment of primary aldosteronism. Langenbecks Arch Surg 2015. [Epub ahead of print]. [PubMed]
- Rossi GP, Pessina AC, Heagerty AM. Primary aldosteronism: an update on screening, diagnosis and treatment. J Hypertens 2008;26:613-21. [PubMed]
- Lumachi F, Marzola MC, Zucchetta P, et al. Non-invasive adrenal imaging in primary aldosteronism. Sensitivity and positive predictive value of radiocholesterol scintigraphy, CT scan and MRI. Nucl Med Commun 2003;24:683-8. [PubMed]
- White ML, Gauger PG, Doherty GM, et al. The role of radiologic studies in the evaluation and management of primary hyperaldosteronism. Surgery 2008;144:926-33. [PubMed]
- Stowasser M, Gordon RD. Primary aldosteronism--careful investigation is essential and rewarding. Mol Cell Endocrinol 2004;217:33-9. [PubMed]
- Griffin AC, Kelz R. LiVolsi VA. Aldosterone-secreting adrenal cortical carcinoma. A case report and review of the literature. Endocr Pathol 2014;25:344-9. [PubMed]
- Doppman JL, Gill JR Jr, Miller DL, et al. Distinction between hyperaldosteronism due to bilateral hyperplasia and unilateral aldosteronoma: reliability of CT. Radiology 1992;184:677-82. [PubMed]
- Harper R, Ferrett CG, McKnight JA, et al. Accuracy of CT scanning and adrenal vein sampling in the pre-operative localization of aldosterone-secreting adrenal adenomas. QJM 1999;92:643-50. [PubMed]
- McAlister FA, Lewanczuk RZ. Primary hyperaldosteronism and adrenal incidentaloma: an argument for physiologic testing before adrenalectomy. Can J Surg 1998;41:299-305. [PubMed]
- Freriks K, Schultze Kool LJ, Timmers HJ, et al. Determining aldosterone in the adrenal veins in order to establish uni- or bilateral aldosterone production in patients with primary hyperaldosteronism. Ned Tijdschr Geneeskd 2007;151:1029-34. [PubMed]
- Colussi G, Catena C, Sechi LA. Spironolactone, eplerenone and the new aldosterone blockers in endocrine and primary hypertension. J Hypertens 2013;31:3-15. [PubMed]
- Choi SH, Kwon TG, Kim TH. Active potassium supplementation might be mandatory during laparoscopic adrenalectomy for primary hyperaldosteronism. J Endourol 2012;26:666-9. [PubMed]
- Fischer E, Hanslik G, Pallauf A, et al. Prolonged zona glomerulosa insufficiency causing hyperkalemia in primary aldosteronism after adrenalectomy. J Clin Endocrinol Metab 2012;97:3965-73. [PubMed]
- Ghose RP, Hall PM, Bravo EL. Medical management of aldosterone-producing adenomas. Ann Intern Med 1999;131:105-8. [PubMed]
- Ori Y, Chagnac A, Korzets A, et al. Regression of left ventricular hypertrophy in patients with primary aldosteronism/low-renin hypertension on low-dose spironolactone. Nephrol Dial Transplant 2013;28:1787-93. [PubMed]
- Catena C, Colussi G, Lapenna R, et al. Long-term cardiac effects of adrenalectomy or mineralocorticoid antagonists in patients with primary aldosteronism. Hypertension 2007;50:911-8. [PubMed]
- Rossi GP, Cesari M, Cuspidi C, et al. Long-term control of arterial hypertension and regression of left ventricular hypertrophy with treatment of primary aldosteronism. Hypertension 2013;62:62-9. [PubMed]
- Giacchetti G, Ronconi V, Turchi F, et al. Aldosterone as a key mediator of the cardiometabolic syndrome in primary aldosteronism: an observational study. J Hypertens 2007;25:177-86. [PubMed]
- Bernini G, Bacca A, Carli V, et al. Cardiovascular changes in patients with primary aldosteronism after surgical or medical treatment. J Endocrinol Invest 2012;35:274-80. [PubMed]
- Marzano L, Colussi G, Sechi LA, et al. Adrenalectomy Is Comparable With Medical Treatment for Reduction of Left Ventricular Mass in Primary Aldosteronism: Meta-Analysis of Long-Term Studies. Am J Hypertens 2014. [Epub ahead of print]. [PubMed]
- Parthasarathy HK, Ménard J, White WB, et al. A double-blind, randomized study comparing the antihypertensive effect of eplerenone and spironolactone in patients with hypertension and evidence of primary aldosteronism. J Hypertens 2011;29:980-90. [PubMed]
- Amar L, Lorthioir A, Azizi M, et al. PROGRESS IN PRIMARY ALDOSTERONISM: Mineralocorticoid antagonist treatment in aldosterone producing adenoma. Eur J Endocrinol 2014. [Epub ahead of print]. [PubMed]
- Sukor N, Kogovsek C, Gordon RD, et al. Improved quality of life, blood pressure, and biochemical status following laparoscopic adrenalectomy for unilateral primary aldosteronism. J Clin Endocrinol Metab 2010;95:1360-4. [PubMed]
- Kline GA, Pasieka JL, Harvey A, et al. Medical or surgical therapy for primary aldosteronism: post-treatment follow-up as a surrogate measure of comparative outcomes. Ann Surg Oncol 2013;20:2274-8. [PubMed]
- Reimel B, Zanocco K, Russo MJ, et al. The management of aldosterone-producing adrenal adenomas--does adrenalectomy increase costs? Surgery 2010;148:1178-85. [PubMed]
- Gagner M, Lacroix A, Bolté E. Laparoscopic adrenalectomy in Cushing's syndrome and pheochromocytoma. N Engl J Med 1992;327:1033. [PubMed]
- Suzuki K, Kageyama S, Ueda D, et al. Laparoscopic adrenalectomy: clinical experience with 12 cases. J Urol 1993;150:1099-102. [PubMed]
- Higashihara E, Tanaka Y, Horie S, et al. A case report of laparoscopic adrenalectomy. Nihon Hinyokika Gakkai Zasshi 1992;83:1130-3. [PubMed]
- Stuart RC, Chung SC, Lau JY, et al. Laparoscopic adrenalectomy. Br J Surg 1995;82:1498-9. [PubMed]
- Brunt LM, Molmenti EP, Kerbl K, et al. Retroperitoneal endoscopic adrenalectomy: an experimental study. Surg Laparosc Endosc 1993;3:300-6. [PubMed]
- Walz MK, Peitgen K, Hoermann R, et al. Posterior retroperitoneoscopy as a new minimally invasive approach for adrenalectomy: results of 30 adrenalectomies in 27 patients. World J Surg 1996;20:769-74. [PubMed]
- Shen WT, Lim RC, Siperstein AE, et al. Laparoscopic vs open adrenalectomy for the treatment of primary hyperaldosteronism. Arch Surg 1999;134:628-31. [PubMed]
- Takeda M, Go H, Imai T, et al. Laparoscopic adrenalectomy for primary aldosteronism: report of initial ten cases. Surgery 1994;115:621-5. [PubMed]
- Lee J, El-Tamer M, Schifftner T, et al. Open and laparoscopic adrenalectomy: analysis of the National Surgical Quality Improvement Program. J Am Coll Surg 2008;206:953-9. [PubMed]
- Steichen O, Zinzindohoué F, Plouin PF, et al. Outcomes of adrenalectomy in patients with unilateral primary aldosteronism: a review. Horm Metab Res 2012;44:221-7. [PubMed]
- Ramírez-Plaza CP, Perales JL, Camero NM, et al. Outpatient laparoscopic adrenalectomy: a new step ahead. Surg Endosc 2011;25:2570-3. [PubMed]
- Henry JF, Sebag F, Iacobone M, et al. Lessons learned from 274 laparoscopic adrenalectomies. Ann Chir 2002;127:512-9. [PubMed]
- Walz MK, Gwosdz R, Levin SL, et al. Retroperitoneoscopic adrenalectomy in Conn's syndrome caused by adrenal adenomas or nodular hyperplasia. World J Surg 2008;32:847-53. [PubMed]
- Constantinides VA, Christakis I, Touska P, et al. Systematic review and meta-analysis of retroperitoneoscopic versus laparoscopic adrenalectomy. Br J Surg 2012;99:1639-48. [PubMed]
- Nigri G, Rosman AS, Petrucciani N, et al. Meta-analysis of trials comparing laparoscopic transperitoneal and retroperitoneal adrenalectomy. Surgery 2013;153:111-9. [PubMed]
- Chen W, Li F, Chen D, et al. Retroperitoneal versus transperitoneal laparoscopic adrenalectomy in adrenal tumor: a meta-analysis. Surg Laparosc Endosc Percutan Tech 2013;23:121-7. [PubMed]
- Fu B, Zhang X, Wang GX, et al. Long-term results of a prospective, randomized trial comparing retroperitoneoscopic partial versus total adrenalectomy for aldosterone producing adenoma. J Urol 2011;185:1578-82. [PubMed]
- Honda K, Sone M, Tamura N, et al. Adrenal reserve function after unilateral adrenalectomy in patients with primary aldosteronism. J Hypertens 2013;31:2010-7. [PubMed]
- Walz MK. Extent of adrenalectomy for adrenal neoplasm: cortical sparing (subtotal) versus total adrenalectomy. Surg Clin North Am 2004;84:743-53. [PubMed]
- Nakada T, Kubota Y, Sasagawa I, et al. Therapeutic outcome of primary aldosteronism: adrenalectomy versus enucleation of aldosterone-producing adenoma. J Urol 1995;153:1775-80. [PubMed]
- Al-Sobhi S, Peschel R, Bartsch G, et al. Partial laparoscopic adrenalectomy for aldosterone-producing adenoma: short-and long-term results. J Endourol 2000;14:497-9. [PubMed]
- Jeschke K, Janetschek G, Peschel R, et al. Laparoscopic partial adrenalectomy in patients with aldosterone-producing adenomas: indications, technique, and results. Urology 2003;61:69-72. [PubMed]
- Fendrich V, Ramaswamy A, Nies C. Hyperaldosteronism persisting after subtotal adrenalectomy. Chirurg 2003;74:473-7. [PubMed]
- Ishidoya S, Ito A, Sakai K, et al. Laparoscopic partial versus total adrenalectomy for aldosterone producing adenoma. J Urol 2005;174:40-3. [PubMed]
- Iacobone M, Citton M, Viel G, et al. Unilateral adrenal hyperplasia: a novel cause of surgically correctable primary hyperaldosteronism. Surgery 2012;152:1248-55. [PubMed]
- Shigematsu K, Yamaguchi N, Nakagaki T, et al. A case of unilateral adrenal hyperplasia being difficult to distinguish from aldosterone-producing adenoma. Exp Clin Endocrinol Diabetes 2009;117:124-8. [PubMed]
- Nishimoto K, Nakagawa K, Li D, et al. Adrenocortical zonation in humans under normal and pathological conditions. J Clin Endocrinol Metab 2010;95:2296-305. [PubMed]
- Gomez-Sanchez CE, Qi X, Velarde-Miranda C, et al. Development of monoclonal antibodies against human CYP11B1 and CYP11B2. Mol Cell Endocrinol 2014;383:111-7. [PubMed]
- Choi M, Scholl U, Yue P, et al. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science 2011;331:768-72. [PubMed]
- Dekkers T, ter Meer M, Lenders JW, et al. Adrenal nodularity and somatic mutations in primary aldosteronism: one node is the culprit? J Clin Endocrinol Metab 2014;99:E1341-51. [PubMed]
- Arnesen T, Glomnes N, Strømsøy S, et al. Outcome after surgery for primary hyperaldosteronism may depend on KCNJ5 tumor mutation status: a population-based study from Western Norway. Langenbecks Arch Surg 2013;398:869-74. [PubMed]
- Rossi GP, Cesari M, Letizia C, et al. KCNJ5 gene somatic mutations affect cardiac remodelling but do not preclude cure of high blood pressure and regression of left ventricular hypertrophy in primary aldosteronism. J Hypertens 2014;32:1514-21. [PubMed]
- Rossi GP, Bolognesi M, Rizzoni D, et al. Vascular remodeling and duration of hypertension predict outcome of adrenalectomy in primary aldosteronism patients. Hypertension 2008;51:1366-71. [PubMed]
- Kitamoto T, Suematsu S, Matsuzawa Y, et al. Comparison of Cardiovascular Complications in Patients with and without KCNJ5 Gene Mutations Harboring Aldosterone-producing Adenomas. J Atheroscler Thromb 2014. [Epub ahead of print]. [PubMed]
- Lumachi F, Ermani M, Basso SM, et al. Long-term results of adrenalectomy in patients with aldosterone-producing adenomas: multivariate analysis of factors affecting unresolved hypertension and review of the literature. Am Surg 2005;71:864-9. [PubMed]
- Sawka AM, Young WF, Thompson GB, et al. Primary aldosteronism: factors associated with normalization of blood pressure after surgery. Ann Intern Med 2001;135:258-61. [PubMed]
- Zarnegar R, Young WF Jr, Lee J, et al. The aldosteronoma resolution score: predicting complete resolution of hypertension after adrenalectomy for aldosteronoma. Ann Surg 2008;247:511-8. [PubMed]
- Utsumi T, Kawamura K, Imamoto T, et al. High predictive accuracy of Aldosteronoma Resolution Score in Japanese patients with aldosterone-producing adenoma. Surgery 2012;151:437-43. [PubMed]