Carcinoma ex pleomorphic adenoma originating from ectopic salivary gland in the neck region: case report
Introduction
Carcinoma ex pleomorphic adenoma (CEPA) is the malignant salivary gland tumor arising from primary or recurrent pleomorphic adenoma cases (1). It is most commonly seen in parotid gland and most commonly (75%) epithelial cells show malignant transformation; rarely epithelial-myoepithelial or pure myoepithelial cells can show malignant transformation (2). CEPA is one of the three malignant mixed tumors; the other two are carcinosarcoma (true malignant mixed tumor); forming by both epithelial and mesenchymal cells’ malignant transformation and metastasizing pleomorphic adenoma; cervical lymph node or distant metastases from benign pleomorphic adenoma (3).
In the differential diagnosis of neck masses congenital, inflammatory, toxic, tumoral, endocrine and systemic causes can be thought. Slow growing, not firmly adhesive to neighbouring tissue masses are mostly benign, whereas malignancy risk increases with age (4). Although primary and metastatic lesions of parotid and submandibular gland can manifest as a neck mass; pleomorphic adenoma or whartin tumors of ectopic salivary gland in the neck region are also encountered (5-8). According to our knowledge; CEPA originating from ectopic salivary gland in the neck region has not been published yet. In this case report we presented the first case of CEPA originating from neck region mimicking benign neck mass in a 72-year-old female patient.
Case
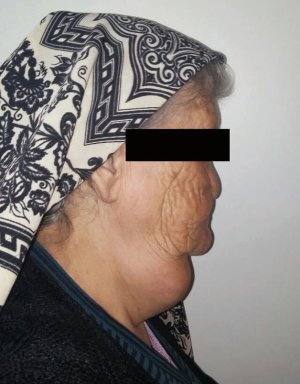
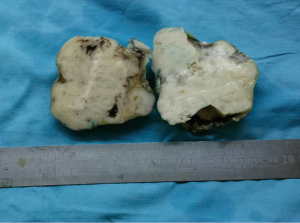
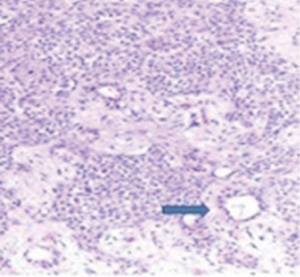
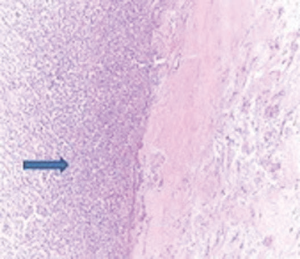
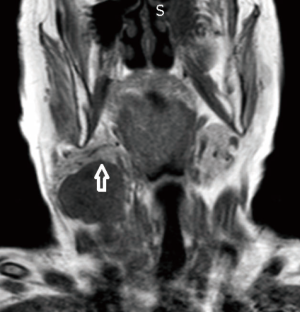
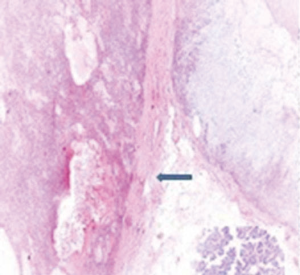
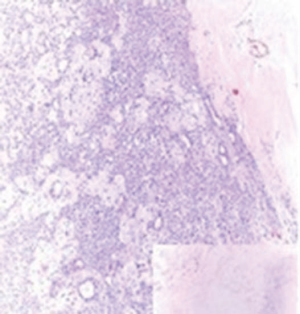
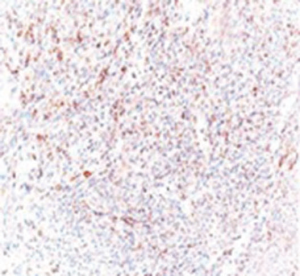
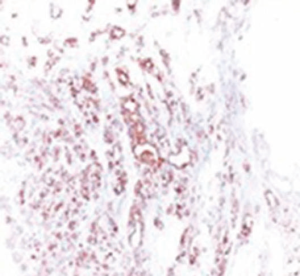
A 72-year-old otherwise healthy female patient admitted to Mersin University otorhinolaryngology department with the complaint of neck mass. The mass was present for 35 years and it was growing slowly, she had no pain complaint. In her medical history, she had no surgery from salivary glands or neck region. In her physical examination, there was a tender on palpation, 7.5 cm mass starting from right submandibular region extending to level 3 and 4 (Figure 1). The mass was mobile on palpation. There was no facial weakness in examination. Examination of oral cavity, oropharynx, indirect endoscopic view of nasopharynx and larynx showed no pathology. In the magnetic resonance imaging (MRI) of the neck, she had a 7.5 cm × 5 cm solid mass extending from submandibular triangle to inferior neck region. The submandibular gland was pushed anteriorly by the mass, but there was a clear boundary between the mass and right submandibular gland (Figure 2). Ultrasound guided fine needle aspiration biopsy of the patient was non-diagnostic, so neck exploration was planned under general anesthesia. Intraoperatively, encapsulated solid mass was totally excised (Figure 3). For excluding malignancy, intraoperatively frozen section was performed. Since result of frozen section analysis was pleomorphic adenoma, right submandibular gland was also included in the surgical specimen. Histopathological examination revealed an intracapsular malign tumor composed of both myoepithelial and epithelial components in a lobular pattern close to normal parotid gland (Figure 4). In addition, the tumor also showed areas of pleomorphic adenoma in the form of admixture of epithelial and stromal elements (Figure 5). The epithelial component was of glandular nature (Figure 6). The tumor includes atypical spindle cells with hyalinized areas (Figure 7). There was no necrosis or lymphovascular and perineural invasion. Immunohistochemistry was positive for smooth muscle antigen (SMA) in myoepithelial area (Figure 8) and epithelial area for cytokeratin (Figure 9). Final histopathological diagnosis was intracapsular epithelial-myoepithelial CEPA. Right submandibular gland had normal morphology pathologically. Since the right submandibular gland was normal and there was a clear boundary between the mass and submandibular gland, intracapsular CEPA was accepted to originate from the ectopic salivary gland tissue in the neck. The tumor was intracapsular form, so neck dissection was not performed; she had no recurrences or another complaint for 1 year follow-up.
Discussion
Salivary gland tumors are rare tumors affecting every 0.4-2.5 patients among 100,000 people each year. A total of 80% are seen in parotid gland and 80% are benign. Pleomorphic adenoma is the most common benign tumor with a mean 67.5% incidence (9). CEPA is the rare malign salivary gland tumor, originating from long lasting or recurrent pleomorphic adenoma (2). It accounts 11.6% of all malignant salivary gland tumors (10). Malignant transformation risk for benign pleomorphic adenoma is 3-15%; increases with time duration after first benign tumor is diagnosed and the size of the tumor (9). In our case; increased size of the tumor as 7.5 cm in long diameter and 35 years long lasting tumor after the first mass was recognized may be the risk factors for malignant transformation. Although exact malignant transformation steps in which pleomorphic adenoma turns into carcinoma is not known; firstly neoplastic luminal cells are thought to be replaced by carcinoma cells. In this stage tumor is surrounded by myoepithelial cells of pleomorphic adenoma called as ‘in situ CEPA’. In the next stage; tumor cells invade beyond the surrounding myoepithelial cells to neighboring stroma, but they are still encapsulated with the capsule of pleomorphic adenoma. This stage is called as ‘intracapsular CEPA’. If invasion continues beyond the fibrous capsule of the pleomorphic adenoma; the diagnosis is invasive CEPA. According to World Health Organization data: if invasion beyond the capsule is limited to 1.5 mm, then it is minimally invasive; if extends beyond 1.5 mm, it is frankly invasive (2,11).
For managing adult patients with neck mass, site of the mass, consistency during palpation, relationship with the neighboring tissue, age of the patient and additional illnesses are important factors. In the differential diagnosis; CITTENS (C-congenital, I-inflammatory, T-toxic, T-traumatic, E-endocrine, N-neoplasms, S-systemic reasons) must be thought (4). In the pediatric population, inflammatory masses are dominant, whereas masses over 40 years of age are much more likely to be neoplastic. Mobile and well-circumscribed masses are mostly thought to be benign in nature (4). In our patient, slowly growing, mobile and well-circumscribed properties of the mass were suggestive of a benign lesion, whereas since age of the patient was 72 years old and FNAB result was non-diagnostic, to exclude malignancy intraoperative frozen section was performed.
Although FNAB has an incontestable role in diagnosis for most of the neck masses, it has limited role for preoperative diagnosis of CEPA (3,9,12). In the series of Lüers et al., only 49% of patients were correctly diagnosed with FNAB, this proportion decreases to 43% in series of Zbären et al., an even to 29% in series of Nouraei et al. (3,9,12). In our patient preoperative FNAB was also non-diagnostic. CEPA can be clinically mimic pleomorphic adenoma preoperatively. Although rapid growing for a long lasting pleomorphic adenoma is suggestive of CEPA, it can also be seen in slowly growing, even 40 years long lasting pleomorphic adenoma cases (1). For our case; CEPA was diagnosed postoperatively in a 35-year long lasting, slow growing, clinically characteristics of a benign mass. Diagnosis of CEPA intraoperatively with frozen section analysis is also disappointing. In the series of Zbären et al., half of the patients were misdiagnosed as pleomorphic adenoma with frozen section most likely due to sampling error, so like clinical characteristics, pathologically pleomorphic adenoma can be mixed with CEPA intraoperatively (3). Since FNAB and frozen section analysis are insufficient, most of the authors argue that mainly the clinical features are important for preoperative diagnosis (3,9,12).
Primary and metastatic lesions of parotid or submandibular gland can be encountered as neck masses. Near these, pleomorphic adenoma or whartin tumors of ectopic salivary gland tissue in the neck region can manifest as a neck mass (5-8). Among malignant salivary gland tumors; cystadenocarcinoma can also originate from ectopic salivary gland tissue in the neck (13). In our case, since intraoperative frozen section analysis revealed pleomorphic adenoma, submandibular gland is also excised. Normal morphology of the gland shows the origin of CEPA in the neck region as ectopic salivary gland tissue, too. CEPA can also be seen in trachea, oral cavity, nasal cavity and lacrimal gland in the head and neck region (1,14-16). Breast tissue; which has nearby morphological properties with salivary glands can also be the origin of CEPA (17). Neck region (de novo) can be added to this list with this case. Also another unique property of our case was that epithelial-myoepithelial cells showed malignant transformation which is seen in only 19% of CEPA cases (2).
Primary treatment of CEPA is surgery. According to site of origin; lateral rhinotomy and medial maxillectomy for lacrimal gland, segmental resection for trachea can be done (14,15,18). Since most of the cases are seen in parotid gland; superficial parotidectomy is sufficient for intracapsular or minimally invasive CEPA whereas total or radical parotidectomy is recommended for frankly invasive cases. Cervical lymph node dissection is advised for clinically obvious neck metastases. Dissection can be functional, modified radical or radical but there is no consensus for the type of the neck dissection (1). Although there is conflicting data for neck dissection and type; since cervical lymph node metastases seems to be an independent predictor of survival, Nouraei et al. recommends routine neck dissection for CEPA cases (12). Lüers et al. argues that neck dissection has both therapeutic and diagnostic meaning in CEPA cases, and only for very small carcinomas with inconspicuous neck metastases, neck dissection may be dispensable (9). The other important factor in prognosis and survival is the pathological subtype of CEPA. Prognosis is excellent with complete excision in cases with intracapsular and in situ CEPA; whereas extended or additional surgery such as radical parotidectomy and neck dissection may be suitable for invasive cases (11). According to our knowledge, there is only one case of neck metastases and one case of lung metastases with benign histological features are present in English literature for intracapsular CEPA (10,19). Since completely excised mass was reported as intracapsular CEPA for our case; she is still in our follow-up without neck dissection for 1 year free of recurrence and metastases.
As a conclusion; for differential diagnosis of long standing neck masses; CEPA of ectopic salivary gland tissue must also be thought.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Antony J, Gopalan V, Smith RA, et al. Carcinoma ex pleomorphic adenoma: a comprehensive review of clinical, pathological and molecular data. Head Neck Pathol 2012;6:1-9. [PubMed]
- Demasi AP, Furuse C, Soares AB, et al. Peroxiredoxin I, platelet-derived growth factor A, and platelet-derived growth factor receptor alpha are overexpressed in carcinoma ex pleomorphic adenoma: association with malignant transformation. Hum Pathol 2009;40:390-7. [PubMed]
- Zbären P, Zbären S, Caversaccio MD, et al. Carcinoma ex pleomorphic adenoma: diagnostic difficulty and outcome. Otolaryngol Head Neck Surg 2008;138:601-5. [PubMed]
- Rosenberg TL, Brown JJ, Jefferson GD. Evaluating the adult patient with a neck mass. Med Clin North Am 2010;94:1017-29. [PubMed]
- Tsakiropoulou E, Konstantinidis I, Vital I, et al. Ectopic Warthin’s tumour presenting as a neck mass. B-ENT 2008;4:111-5. [PubMed]
- Wang MC, Tsai TL, Chen PC, et al. Extraparotid Warthin’s tumor presented as a neck mass. J Chin Med Assoc 2003;66:752-4. [PubMed]
- Luksić I, Suton P, Manojlović S, et al. Pleomorphic adenoma in ectopic salivary gland tissue in the neck. Coll Antropol 2012;36:133-6. [PubMed]
- Arunkumar KV, Kumar S, Bansal V, et al. Pleomorphic adenoma--unusual presentation of a salivary gland tumor in the neck of a child. Quintessence Int 2011;42:879-82. [PubMed]
- Lüers JC, Wittekindt C, Streppel M, et al. Carcinoma ex pleomorphic adenoma of the parotid gland. Study and implications for diagnostics and therapy. Acta Oncol 2009;48:132-6. [PubMed]
- Felix A, Rosa-Santos J, Mendonça ME, et al. Intracapsular carcinoma ex pleomorphic adenoma. Report of a case with unusual metastatic behaviour. Oral Oncol 2002;38:107-10. [PubMed]
- Cheuk W, Chan JK. Advances in salivary gland pathology. Histopathology 2007;51:1-20. [PubMed]
- Nouraei SA, Hope KL, Kelly CG, et al. Carcinoma ex benign pleomorphic adenoma of the parotid gland. Plast Reconstr Surg 2005;116:1206-13. [PubMed]
- Enomoto K, Yamashita H, Harada H, et al. A case of cystadenocarcinoma of the ectopic salivary gland: comparison of pre-operative ultrasound, CT and MR images with the pathological specimen. Dentomaxillofac Radiol 2012;41:349-54. [PubMed]
- Ding CS, Yap WM, Teo CH, et al. Tracheal carcinoma ex pleomorphic adenoma: a rare tumour with potential problems in diagnosis. Histopathology 2007;51:868-71. [PubMed]
- Demirağ F, Topçu S, Kurul C, et al. Malignant pleomorphic adenoma (malignant mixed tumor) of the trachea: a case report and review of the literature. Eur Arch Otorhinolaryngol 2003;260:96-9. [PubMed]
- Hong HJ, Byeon HK, Bae SH, et al. Carcinoma ex pleomorphic adenoma in the oral cavity: a huge oral cavity mass with neck metastasis. J Craniofac Surg 2013;24:e543-6. [PubMed]
- Hayes MM, Lesack D, Girardet C, et al. Carcinoma ex-pleomorphic adenoma of the breast. Report of three cases suggesting a relationship to metaplastic carcinoma of matrix-producing type. Virchows Arch 2005;446:142-9. [PubMed]
- Baredes S, Ludwin DB, Troublefield YL, et al. Adenocarcinoma ex-pleomorphic adenoma of the lacrimal sac and nasolacrimal duct: a case report. Laryngoscope 2003;113:940-2. [PubMed]
- Weissferdt A, Langman G.. An intracapsular carcinoma ex pleomorphic adenoma with lung metastases composed exclusively of benign elements: histological evidence of a continuum between metastasizing pleomorphic adenoma and carcinoma ex pleomorphic adenoma. Pathol Res Pract 2010;206:480-3. [PubMed]