Detection of insulinoma using 68Gallium-DOTATATE PET/CT: a case report
Introduction
Insulinomas are the most common cause of endogenous hyperinsulinemic hypoglycemia in nondiabetic adult patients, with an incidence of 1-3 per million per year (1). They are most commonly localized in the pancreas and approximately 85% are solitary, 6-13% are multiple and 4-6% are associated with multiple endocrine neoplasia type 1 (1,2). More than 90% of insulinomas are benign.
The clinical features of insulinoma result from hypoglycemia and autonomic nervous overactivity. Patients may present with the Whipple triad: symptoms of hypoglycemia, low blood glucose (40-50 mg/dL), and relief of symptoms after the administration of glucose (3). The gold standard for the diagnosis of insulinoma is a 48-hour supervised fast when the insulin/proinsulin levels are inappropriately high for a glucose level below 40 mg/dL with negative levels for beta-hydroxybutyrate and sulfonylurea and using a point of discrimination for proinsulin of ≥22 pmol/L (4). Seventy-nine percent of patients with insulinomas will develop hypoglycemic symptoms within 24 hours and 100% within 48 hours (4,5).
Once a biochemical diagnosis of an insulinoma is established, localization procedures are performed. Due to their small size (82% <2 cm, 47% <1 cm), insulinomas are difficult to localize (1). Ultrasound (US), computed tomography (CT) and magnetic resonance imaging (MRI) are widely available and are noninvasive localizing studies. However, their accuracy for localizing tumors is poor (positive in <50% of cases) (6). Endoscopic US is positive in 70-95% of cases but often misses lesions in the tail of the pancreas, and is operator dependent (7). Selective angiography can detect lesions in approximately 60% of patients and intra-arterial calcium stimulation with hepatic venous sampling for insulin levels localizes >80% of insulinomas, but it is invasive, costly, requires an experienced interventional radiologist, and only regionalizes the tumor (6,8).
Even when localizing studies are negative, all patients should undergo surgical exploration, with intraoperative US of the pancreas and manual palpation. Positron emitting radiopharmaceuticals for somatostatin receptor (SSTR) imaging, DOTA analogs, have been developed and show promising results, with high accuracy in detecting primary, recurrent, and metastatic tumors as compared to anatomic imaging, traditional radiopharmaceuticals, and endoscopy for gastrointestinal and pancreatic neuroendocrine tumors (NETs). These radioligands, which include 68Gallium-DOTATATE, 68Gallium-DOTATOC, and 68Gallium-DOTANOC, have a high affinity to SSTR, especially to SSTR2. A comparison of this new SSTR imaging in a meta-analysis study suggested that 68Gallium-DOTATATE was most accurate for detecting NETs (9). Recently, several studies have demonstrated that 68Gallium-labeled somatostatin analog positron emission tomography (PET) when combined with CT has a higher sensitivity for detecting NETs than SSTR scintigraphy (10,11). In this report, we describe one patient in whom 68Gallium PET/CT using the analogue DOTATATE localized a solitary, benign insulinoma with correlative immunohistochemical analysis.
Methods
Patients with a diagnosis of insulinoma at the National Institutes of Health (NIH) have noninvasive localization studies including transabdominal US, pancreatic protocol CT, and MRI. Arterial calcium stimulation with hepatic vein catheterization is also performed, which uses intravenous calcium as a secretagogue for the release of insulin from the islet cell tumors. The gastroduodenal, proper hepatic, superior mesenteric artery (SMA) and splenic arteries are selectively catheterized with calcium gluconate bolused into the selected artery. Blood samples from the right and left hepatic veins are obtained before and 20, 40, and 60 s after injection. A ≥2-fold increase in insulin concentration from baseline localizes the insulinoma within the anatomic region perfused by the injected artery. A response after calcium infusion into the gastroduodenal or SMA localizes the lesion to the head and neck, whereas a response after splenic artery injection localizes the lesion to the body and tail of the pancreas. A response following a hepatic artery injection suggests the presence of liver metastases (6,12).
68Gallium-DOTATATE PET/CT is considered investigational and is not approved for routine use to localize NETs in the United States; therefore the current study was performed under a research protocol approved by the NIH Review Board and (NCT01967537). Through an antecubital vein, 5 mCi of 68Ga-DOTATATE is administered. After 60 minutes, the patient is positioned in a PET/CT scanner and images from the upper thighs to the base of the skull are obtained. A non-contrast, non-diagnostic CT is used for attenuation and anatomic localization. Maximum standardized uptake values (SUV) are measured based on patient total body weight. An 111In- pentetreotide SPECT/CT with imaging at 4 h and at 24 h following intravenous administration of 6 mCi (222 MBq) of 111In pentetreotide was performed.
For immunohistochemistry, primary antibody for SSTR2 [(UMB1) ab134152, Abcam, Cambridge, MA] and SSTR5 [(UMB4) ab109495, Abcam] was used at 1:60 dilution.
Case
A 40-year-old man with a longstanding history of seizures associated with hypoglycemia was referred to our institution. He had no significant past medical history. Physical examination was normal. The 48-hour supervised fast was stopped at 6 hours for neuroglycopenic symptoms with a blood glucose level of 33 mg/dL (reference range, 60-100 mg/dL). Symptoms resolved with administration of dextrose. The corresponding insulin was 12.9 mcU/mL (reference range, 2.6-24.9 mcU/mL), proinsulin was 130 pmol/L [point of discrimination ≥22 pmol/L (4)], C-peptide was 2.6 ng/mL (reference range, 1.1-5 ng/mL), and sulfonylurea screening was negative. His calcium level was normal at 2.35 mmol/L (reference range, 2.05-2.5 mmol/L) and his family history was negative for primary hyperparathyroidism or NETs.
An endoscopic US of the pancreas performed at another institution did not show any lesions. CT scan and MRI of the pancreas revealed a 2 cm lobulated lesion on the anterior aspect of the distal body (Figure 1). An arteriogram with selective arterial calcium stimulation and venous sampling, showed an arterial blush in the anterior aspect of the pancreatic body and increased insulin level with calcium-stimulation in the mid splenic at 20 s (Figure 1E,F). A 68Gallium-DOTATATE PET/CT scan showed uptake in the anterior aspect of the distal body of the pancreas (Figure 1B,C). 111Indium-pentetreotide scan and abdominal US were negative (Figure 1A).
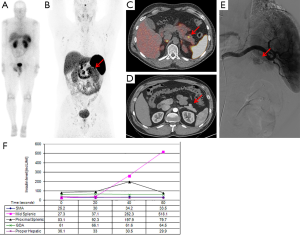
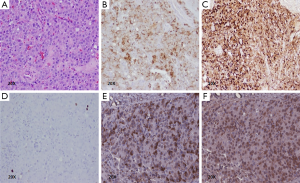
The patient underwent a laparoscopic exploration and intraoperative US of the pancreas which showed a solitary, hypoechoic, vascular tumor on the anterior surface of the distal body of the pancreas. The tumor was enucleated. Pathology showed a 2.1 cm × 1.6 cm × 1.5 cm low grade (proliferative index Ki-67 staining <2%), well-differentiated pancreatic NET that was positive for insulin, SSTR2 and SSTR5 expression (Figure 2). The patient remains euglycemic and free of symptoms 3.5 months after his operation.
Discussion
We report a case of benign, sporadic insulinoma that was detected for the first time using the radiotracer 68Gallium-DOTATATE. In the United States, the use of 68Gallium-DOTATATE PET/CT is considered investigational and is not approved for routine use to localize NETs. The patient was evaluated on a prospective clinical protocol comparing the accuracy of anatomic imaging (CT), 111Indium-pentetreotide scan and 68Gallium-DOTATATE PET/CT. As shown in Figure 1, 68Gallium-DOTATATE PET/CT provides anatomic information on the exact location of the lesion that shows DOTATATE avidity, with an SUV of 18.5.
The management of insulinomas is challenging and requires a multidisciplinary approach for diagnosis and localization. Although most insulinomas are <2 cm, our patient had a relatively large tumor (2.1 cm) which was detected by anatomic imaging. It has been previously reported that 68Gallium-DOTATATE PET/CT can detect NETs as small as 6 mm (10,13). The sensitivity of 111Indium-pentetreotide scintigraphy to detect insulinomas is lower than for most gastrointestinal NETs, sensitivity of 20-60% (14-16), even though insulinomas are reported to express SSTR2 and 5 (17). These receptors have been successfully targeted in gastrointestinal and pancreatic NETs with 68Gallium-DOTATATE, at a comparable diagnostic accuracy as other tracers such as DOTANOC (11,18). These findings suggest that 68Gallium-DOTATATE has high affinity for SSTR2 and SSTR5 and thus when positive, are specific for NETs in the pancreas as observed in our patient. Another recently described radioligand to detect insulinomas is 111In-labelled exendin-4 which displays a high sensitivity for insulinomas because it targets the glucagon-like peptide 1 receptor, which is highly expressed in insulinomas (19-21).
Although the diagnosis of insulinoma is a biochemical diagnosis, localization of these tumors before surgical treatment can facilitate the type of surgical procedure used and improves the likelihood of successful surgical treatment. At our institution, patients undergo multimodal localizing studies. All of these localizing studies should be considered complementary in the management of patients with insulinoma.
In conclusion, 68Gallium-DOTATATE PET/CT could be a helpful adjunct localizing study to detect insulinomas, especially when other localizing studies such as endoscopic US and calcium stimulation are unrevealing. A larger study is needed to fully assess the accuracy of 68Gallium-DOTATATE PET/CT in patients with insulinoma as compared to current localizing studies used in clinical practice.
Acknowledgements
This research was supported by the intramural research program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
Disclosure: The authors declare no conflict of interest.
References
- Service FJ, McMahon MM, O’Brien PC, et al. Functioning insulinoma--incidence, recurrence, and long-term survival of patients: a 60-year study. Mayo Clin Proc 1991;66:711-9. [PubMed]
- Grant CS. Insulinoma. Best Pract Res Clin Gastroenterol 2005;19:783-98. [PubMed]
- Whipple AO, Frantz VK. Adenoma of islet cells with hyperinsulinism: a review. Ann Surg 1935;101:1299-335. [PubMed]
- Guettier JM, Lungu A, Goodling A, et al. The role of proinsulin and insulin in the diagnosis of insulinoma: a critical evaluation of the Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2013;98:4752-8. [PubMed]
- Hirshberg B, Livi A, Bartlett DL, et al. Forty-eight-hour fast: the diagnostic test for insulinoma. J Clin Endocrinol Metab 2000;85:3222-6. [PubMed]
- Guettier JM, Kam A, Chang R, et al. Localization of insulinomas to regions of the pancreas by intraarterial calcium stimulation: the NIH experience. J Clin Endocrinol Metab 2009;94:1074-80. [PubMed]
- McLean A. Endoscopic ultrasound in the detection of pancreatic islet cell tumours. Cancer Imaging 2004;4:84-91. [PubMed]
- Brown CK, Bartlett DL, Doppman JL, et al. Intraarterial calcium stimulation and intraoperative ultrasonography in the localization and resection of insulinomas. Surgery 1997;122:1189-93; discussion 1193-4. [PubMed]
- Yang J, Kan Y, Ge BH, et al. Diagnostic role of Gallium-68 DOTATOC and Gallium-68 DOTATATE PET in patients with neuroendocrine tumors: a meta-analysis. Acta Radiol 2014;55:389-98. [PubMed]
- Gabriel M, Decristoforo C, Kendler D, et al. 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: comparison with somatostatin receptor scintigraphy and CT. J Nucl Med 2007;48:508-18. [PubMed]
- Haug AR, Cindea-Drimus R, Auernhammer CJ, et al. The role of 68Ga-DOTATATE PET/CT in suspected neuroendocrine tumors. J Nucl Med 2012;53:1686-92. [PubMed]
- Doppman JL, Chang R, Fraker DL, et al. Localization of insulinomas to regions of the pancreas by intra-arterial stimulation with calcium. Ann Intern Med 1995;123:269-73. [PubMed]
- Srirajaskanthan R, Kayani I, Quigley AM, et al. The role of 68Ga-DOTATATE PET in patients with neuroendocrine tumors and negative or equivocal findings on 111In-DTPA-octreotide scintigraphy. J Nucl Med 2010;51:875-82. [PubMed]
- Krenning EP, Kwekkeboom DJ, Bakker WH, et al. Somatostatin receptor scintigraphy with [111In-DTPA-D-Phe1]- and [123I-Tyr3]-octreotide: the Rotterdam experience with more than 1000 patients. Eur J Nucl Med 1993;20:716-31. [PubMed]
- Schillaci O, Massa R, Scopinaro F. 111In-pentetreotide scintigraphy in the detection of insulinomas: importance of SPECT imaging. J Nucl Med 2000;41:459-62. [PubMed]
- de Herder WW, Niederle B, Scoazec JY, et al. Well-differentiated pancreatic tumor/carcinoma: insulinoma. Neuroendocrinology 2006;84:183-8. [PubMed]
- Bertherat J, Tenenbaum F, Perlemoine K, et al. Somatostatin receptors 2 and 5 are the major somatostatin receptors in insulinomas: an in vivo and in vitro study. J Clin Endocrinol Metab 2003;88:5353-60. [PubMed]
- Kabasakal L, Demirci E, Ocak M, et al. Comparison of 68Ga-DOTATATE and 68Ga-DOTANOC PET/CT imaging in the same patient group with neuroendocrine tumours. Eur J Nucl Med Mol Imaging 2012;39:1271-7. [PubMed]
- Christ E, Wild D, Forrer F, et al. Glucagon-like peptide-1 receptor imaging for localization of insulinomas. J Clin Endocrinol Metab 2009;94:4398-405. [PubMed]
- Eriksson O, Velikyan I, Selvaraju RK, et al. Detection of metastatic insulinoma by positron emission tomography with [(68)ga]exendin-4-a case report. J Clin Endocrinol Metab 2014;99:1519-24. [PubMed]
- Christ E, Wild D, Ederer S, et al. Glucagon-like peptide-1 receptor imaging for the localisation of insulinomas: a prospective multicentre imaging study. Lancet Diabetes Endocrinol 2013;1:115-22. [PubMed]


