Update on pancreatic neuroendocrine tumors
Introduction
Neuroendocrine tumors (NETs) are a diverse group of neoplasms that can arise from a variety of different organs. Commonly found in the gastrointestinal (GI) tract and lung, they also arise in the pancreas. Historically, NETs of the GI tract and pancreatic neuroendocrine tumors (pNETs) were placed in the same category, however, pNETs exhibit important differences and are best considered separately. Poorly understood for many years, there have been a number of recent advances in our understanding of these tumors. This review will summarize the epidemiology, pathology, diagnosis, and management of this disease, including how recent discoveries in the molecular changes these tumors manifest is changing therapy.
Epidemiology
Originally called islet cell tumors, pNETs were re-designated by the World Health Organization (WHO) in 2010. These tumors are rare, with an incidence of 0.43 per 100,000 according to the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) registry; however, this rate has more than doubled in the last 20-30 years (1,2). This increase is due, at least in part, to increased physician awareness, improvements in diagnostic imaging, and the overall increased use of CT scans. However, autopsy studies have found the prevalence of pNETs ranges from 0.8% to 10% (3-5), suggesting that the vast majority of them are clinically silent. There is a slight male predominance (55% male vs. 45% female). Most patients present in their 50s, although patients with functional tumors present earlier than patients with non-functional tumors (mean age of presentation 55 vs. 59 years) (6). The vast majority of these patients are Caucasian (84% vs. 16%) (3). Overall, pNETs comprise 1-2% of all pancreatic tumors and 7% of NETs in general, second only to gastrointestinal carcinoid (7-10).
Syndromic pNETS
The majority of pNETs arise sporadically, but approximately 10% are associated with an underlying genetic syndrome such as multiple endocrine neoplasia type I (MEN1) and type IV (MEN4), von Hippel-Lindau disease (VHL), neurofibromatosis type I (NF1), or tuberous sclerosis complex (TSC) (11). pNETs are most prevalent in MEN1 with nearly all individuals having multiple non-functional adenomas on autopsy, predominantly microadenomas (<5 mm in diameter) (11,12). The prevalence of clinically significant pNETs increases with age with roughly 50% of patients being diagnosed by age 50, and most having multiple pNETs (12-15).
The most common type of pNET in MEN1 is a non-functional tumor, however, the majority of MEN1 patients will develop symptomatic lesions, with around 50% developing Zollinger-Ellison (ZE) syndrome from an underlying gastrinoma, roughly 20% developing symptoms of an insulinoma, and 3-5% developing VIPomas or glucagonomas (8,11-17). Overall, the result is that 25% of all gastrinomas, and 4% of all insulinomas are linked to MEN1 (8). Patients with MEN1 will also develop NETs in other organs; however, development of a pNET is a poor prognostic factor. pNETs are the most common underlying cause of MEN1 associated mortality, and these patients have a reduced life expectancy of 69 years compared to 77 years for MEN1 patients without a pNET (11,15,18).
Recently, MEN4, which is caused by a mutation in CDKN1B which codes for p27, was discovered in a subset of patients with MEN1-like syndromes without identifiable MEN1 mutations. These patients are prone to MEN1 associated tumors in addition to adrenal, renal, gonadal, and thyroid tumors. Given its recent discovery, the prevalence and natural history of pNETs in this genetic syndrome is unclear (19,20).
Pancreatic neoplasms in general are common in VHL with 35-77% of patients developing lesions. The majority of these are benign, with serous cystadenomas being the most common. Only 10-17% of patients with VHL will develop a true pNET, almost always non-functional (8,11). The incidence of pNET in NF1 is 0-10%, comprised of almost exclusively somatostatinomas. pNETs are also rare in TSC, with an incidence of <1%, usually associated with TSC type I (8,11).
Pathogenesis
The endocrine function of the pancreas is carried out by pockets of cells designated as the islets of Langerhans. These cells secrete many key hormones, including insulin, glucagon, somatostatin, vasoactive intestinal peptide (VIP), and others. These are hormones commonly produced by functional pNETs. Originally, it was thought that pNETs arose from the islets of Langerhans, but more recent investigation has suggested they arise from pluripotent stem cells in the pancreatic ductal/acinar system (21). However, pNETs demonstrate important genetic differences from pancreatic adenocarcinomas. Recent pNET exome sequencing demonstrated that gene mutations typical for adenocarcinoma, such as KRAS, are absent in pNETs. Furthermore, pNETs appear to commonly involve distinct mutations from adenocarcinoma, particularly MEN1 in 44% of tumors, DAXX in 25% of tumors, ATRX in 18% of tumors, and mTOR pathway genes in 16% of tumors (22).
MEN1 codes for menin, which has an essential function in chromatin remodeling regulation, and its role in NET development has long been known due to its involvement in the MEN1 syndrome. The serine-threonine kinase mTOR is at the center of an oncogenic pathway involved in cell growth and proliferation (22). Interestingly DAXX and ATRX mutations have not been previously associated with cancer. DAXX and ATRX participate in chromatin remodeling at telomeres and other genomic sites, and appear to be associated with pNETs through development of a telomerase-independent telomere maintenance process termed alternative lengthening of telomeres (ALT) (23-26). The ALT phenotype is found in immortalized cell lines and has been implicated in some human cancers. In one series, 61% of pNETs had abnormal telomeres consistent with ALT and there was a perfect correlation between these tumors and DAXX/ATRX mutations or loss of nuclear expression of these genes (23). Loss of DAXX/ATRX and development of ALT appear to occur late in pNET development, being found in larger tumors (>2-3 cm) and metastatic lymph nodes (24). DAXX/ATRX mutations are also a poor prognostic sign and are associated with earlier recurrence and decreased disease specific survival (25). Furthermore, the DAXX/ATRX pathway appears to be unique to pNETs among other gastrointestinal NETs. In one series of gastrointestinal carcinoid tumors, only 4% demonstrated the ALT phenotype, and the presence of ALT has been proposed as a method to predict the site of origin of NET liver metastases with unknown primary (26). These recent discoveries, particularly the mutations in the mTOR pathway genes, suggest exciting possibilities for targeted molecular therapy.
Pathology/staging
The history of classification and staging of pNETs is complex and has undergone a great number of changes in the last 10-15 years. Currently, the WHO, European Neuroendocrine Tumor Society (ENETS), and American Joint Committee on Cancer (AJCC) have each proposed a formal staging system for pNETs (27-29). The 2010 WHO classification system is based on the proliferative activity of the tumor as measured by mitotic count and the expression of nuclear antigen Ki-67, a marker for cellular proliferation. Grade 1 tumors have fewer than 2 mitoses per 10 high power fields and less than or equal to 3% Ki-67 staining. Grade 2 tumors have 2-10 mitoses per 10 high power fields or 3-20% Ki-67 staining. Grade 3 tumors have greater than 20 mitoses per 10 high power fields or greater than 20% Ki-67 staining. Grade 1 and 2 lesions are well differentiated and classified as NETs (90% of tumors), while Grade 3 lesions are poorly differentiated and classified as neuroendocrine carcinomas (10% of tumors). The WHO classification system is summarized in Table 1 (27).

Full table
The ENETS staging system is based on TNM classification (Table 2) while the AJCC staging system is taken from the TNM staging system developed for pancreatic adenocarcinoma (Table 3) (28,29). Most head to head comparisons of the ENETS and the AJCC staging systems have shown no statistical difference in their ability to predict survival (30-32), however, one cohort study did find a slight advantage favoring the ENETS system (33). In the face of three different staging systems, work towards a single, comprehensive, accurately predictive model continues. Recently, one retrospective analysis suggested that combining the WHO grading system using Ki-67 expression rates with the lesser known Hochwald grading system, which divides tumors into two stages based on tumor necrosis and mitotic rates, is more predictive of survival than any of the current staging systems (32). Similarly, another series out of Johns Hopkins found that Ki-67 expression rates have a linear relationship with mortality, calling into question the validity of breaking Ki-67 rates into categories as the WHO system does. They proposed a nomogram based on age, gender, and Ki-67 labeling as a continuous variable that appears to be both simpler and more prognostic than either the ENETS or AJCC systems (30). The staging of pNETs likely will continue to evolve in coming years as our understanding increases.
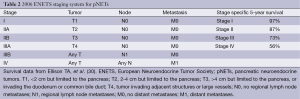
Full table
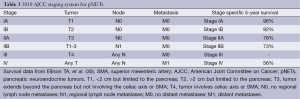
Full table
Presentation
pNETs are divided into functional versus non-functional tumors with about 90% being classified as non-functional. Commonly, tumors are defined as non-functional if the patient does not suffer from symptoms due to hormone hyper-secretion, even if hormone levels are elevated on laboratory evaluation. Most non-functional pNETs present with symptoms due to mass effect, such as jaundice, weight loss, abdominal pain, palpable mass, nausea/emesis, pancreatitis, or back pain, and mimic the presentation of pancreatic adenocarcinoma. As routine diagnostic imaging becomes more prevalent, some patients present asymptomatically with an incidental finding on cross sectional imaging. Most patients present with metastatic (60%) or locally advanced disease (21%) (3).
As mentioned before, pNETs are functional 10% of the time. The presenting symptoms of functional tumors depend on the particular hormone that is being overproduced. The most common functional pNETs are insulinomas, gastrinomas, VIPomas, glucagonomas, and somatostatinomas. Insulinomas comprise 35-40% of all functional pNETs, and present with symptoms of episodic hyperinsulinemia classically referred to as Whipple’s triad: symptoms of hypoglycemia (weakness, sweating, tremors, palpitations, confusion, visual changes etc.) during fasting or exercise, documented hypoglycemia at time of symptoms, and symptom resolution with glucose administration (8,11). Gastrinomas make up 16-30% of functional pNETs and hyper-secrete gastrin resulting in Zollinger Ellison syndrome, the classic symptoms of which are refractory peptic ulcer disease and secretory diarrhea (8,11).
Glucagonomas or VIPomas each account for less than 10% of functional pNETs (8,11). The most common presenting symptom of glucagonomas is a dermatitis called migratory necrolytic erythema, consisting of erythematous lesions that become necrotic and develop pigmented scarring (34). Other common symptoms from glucagonomas include glucose intolerance, weight loss, diarrhea, and deep vein thrombosis (DVT). Together these symptoms are sometimes referred to as the 4D syndrome (dermatitis, diabetes, diarrhea, DVT) (8,11). VIPomas secrete vasoactive intestinal polypeptide and result in symptoms of large volume watery diarrhea and hypokalemia. Somatostatinomas comprise less than 5% of pNETs. They secrete somatostatin and can cause diabetes mellitus, gallbladder disease, diarrhea/steatorrhea, anemia, and weight loss. Somatostatinomas have the subtlest syndrome of any of the functional pNETs and rarely is the syndrome present in its entirety in a single patient (8,11,35). The various syndromes associated with functional pNETs are summarized in Table 4.

Full table
Diagnosis/staging
History and physical
A detailed history and physical is essential in these patients. The history should focus on signs of mass effect or metastasis, evaluate for symptoms of an endocrine syndrome, and screen for family history suggestive of genetic syndromes associate with pNETs. Physical exam should look for jaundice, and abdominal masses.
Laboratory evaluation
If a functional tumor is suspected, workup should include biochemical assessment for the appropriate syndrome. Seventy two-hour fast is the gold standard for diagnosis of an insulinoma, with measurement of glucose and insulin levels at the time of symptoms. It is also important to measure C-peptide to rule out surreptitious insulin use (36). With gastrinoma, an elevated fasting serum gastrin level is usually the first test and a level greater than ten times the limits of normal is virtually diagnostic of this disease. Proton pump inhibitors elevate serum gastrin levels, and it is important to draw labs after holding these drugs for one week due to their long acting nature. ZE syndrome is usually confirmed with a secretin stimulation test, but gastric acid secretion studies are sometimes required (8,37-40). Migratory necrotizing dermatitis, while highly suggestive of a glucagonoma, can also occur in celiac disease, cirrhosis, or pancreatitis, and the diagnosis must be confirmed by elevated glucagon levels (34,41,42). VIPoma and somatostatinoma are confirmed by elevated levels of VIP and somatostatin respectively (43,44).
A variety of tumor markers have been proposed for functional and non-functional pNETs. The most common of these is chromogranin A (CgA), an acid soluble protein that is found in secretory granules of neuroendocrine cells, although others, such as neuron-specific enolase (NSE), pancreatic polypeptide, pancreastatin, and human chorionic gonadotropin have been proposed. CgA is the most sensitive of these, with elevated levels present in 72-100% of patients. However, CgA levels are highly variable, limiting specificity to 50-80% (45-47). Furthermore, proton-pump inhibitor use, impaired renal function, liver disease, and inflammatory bowel disease can all cause an increase in CgA leading to false positive results. Higher CgA levels correlate with increased tumor burden and metastatic disease, and may be most useful in assessing response to therapy (45-47). The sensitivity of NSE as a tumor marker is low at 30-40%, but its specificity is almost 100% (48). Using a combination of CgA and NSE levels improves the sensitivity of using either alone (49).
Imaging
Localization and staging of the tumor is essential to appropriate therapy for pNET. A variety of imaging modalities exist to assist the clinician, including computed tomography (CT), magnetic resonance imaging (MRI), somatostatin receptor scintigraphy (SRS), positron-emission tomography (PET), and endoscopic ultrasonography (EUS). In the rare case that the tumor cannot be located with these modalities, angiography with selective arterial stimulation and venous sampling may be employed. If the tumor cannot be located prior to surgery, bimanual palpation with intra-operative ultrasound often discovers the lesion as a last resort. This is a situation seen most often with small insulinomas that are only a few millimeters in size.
CT
CT is the most common initial imaging study in the evaluation of patients with pNETs. Triple-phase contrast CT is the optimal study as pNETs are typically best visualized during the arterial phase. They usually appear as spherical, hyper-dense, and hyper-vascular mass that rarely obstruct the pancreatic duct. The reported sensitivity of CT ranges from 62-83% with a specificity of 83-100%, although it varies with the size of the lesion (50,51). Although most pNETs are solid lesions, about 10% will present as a cystic lesions with smooth margins and peripheral enhancement on both arterial and portal phases. Overall, it is difficult to differentiate cystic pNETs from other cystic pancreatic lesions on cross-sectional imaging with a misdiagnosis rate of 43% in a recent series (52).
MR
pNETs are usually well visualized on MR. The MR signal is typically low in T1-weighted sequences, and high in T2-weighted sequences. Again, pNETs are best visualized during the arterial contrast phase. The sensitivity of MR ranges from 85-100% with a specificity of 75-100% (50,53). In one recent series of 55 patients, the sensitivity of MR was 95%, rivaling that of EUS (54). Not as commonly used as CT, MR is most often ordered when lesions are too small to be visualized on CT. In detecting and following liver metastases, MR has been suggested to be superior to CT (53,55,56).
SRS
SRS uses radiolabeled somatostatin analogs and relies on somatostatin receptors expressed by pNETs. This leads to an important caveat; that insulinomas, in which somatostatin receptors are present only at low levels or absent entirely, are not well visualized with this technique. However, for other functional pNETs and nonfunctional pNETs the ability of SRS to localize the tumor is good, with sensitivities ranging from 75-100% (53,57). SRS is often used when a functional pNET is suspected and conventional cross-sectional imaging fails to localize the tumor. It can be particularly helpful with glucagonoma as these have a greater propensity to present outside of the pancreas than other functional NETs (50). SRS has an advantage over other imaging modalities in evaluating patients for sufficient uptake for targeted radiation therapy using radiolabeled somatostatin analogs. SRS is also typically useful in evaluating the burden of metastatic disease.
PET
Standard PET imaging with 18F-Fluorodeoxyglucose (FDG) does not visualize pNETs well, given that most pNETs are well differentiated with a low metabolic rate. However, it can detect poorly differentiated pNETs and FDG avidity correlates with early tumor progression and increased mortality (58,59). Alternatively, PET imaging has increasingly utilized 68Ga labeled somatostatin analogs with excellent results. PET imaging with this utilization has been shown to be superior to both SRS and conventional cross-sectional imaging (60-67). The results from fusion of PET with CT images are better than either modality individually with sensitivities of 94-100% (68). In one series, use of fused PET/CT images changed treatment decisions in 59.6% of patients compared to CT or MRI alone (63).
EUS
EUS has become an invaluable tool in the evaluation of pancreatic lesions. In addition to radiologic examination of the pancreas, EUS offers the additional benefit of obtaining biopsies for diagnosis. EUS has an 82% sensitivity and a 92% specificity in identifying pNETs, although EUS is more sensitive in the head of the pancreas than the tail and results are operator dependent (69,70). EUS is most useful in identifying small insulinomas, as these lesions infrequently express somatostatin receptors and are not well visualized on SRS or PET. EUS has the added benefit of being able to tattoo smaller lesions for easier intraoperative identification, facilitating laparoscopic resection (71).
Treatment
As mentioned before, accurate staging of pNETs is essential for determining appropriate therapy. Lesions should be surgically resected when possible, as this is the only potentially curative therapy. Most patients, however, present with metastatic disease. The goal of therapy in advanced disease is to palliate the symptoms of hormone excess in functional tumors and lengthen survival. This requires a multidisciplinary approach including cytoreductive surgery when appropriate, directed therapy for the treatment of liver metastases when possible, and systemic medical therapy.
Surgically resectable disease
Local disease is treated with surgical removal of the tumor. Possible operations include simple enucleation, distal pancreatectomy with splenectomy, spleen-preserving distal pancreatectomy, central pancreatectomy, pancreaticoduodenectomy, and total pancreatectomy. Historically, enucleation has been reserved for insulinomas and small, less than 2 cm, non-functional pNETs that are distant from the pancreatic duct. However, the use of enucleation in non-insulinoma pNETs has been called into question. There are few studies comparing enucleation to formal pancreatic resections head to head. Those that exist generally find an advantage in operative time, blood loss, post-operative endocrine/exocrine pancreatic function, and hospital/ICU stay in the enucleation group. These studies also find equivalent 5- and 10-year survival rates between the two groups (72-75). The data regarding morbidity are conflicting. The most common complication following enucleation is development of a pancreatic fistula (PF). Some studies find no difference in PF rates (72,74-76), while others report a higher incidence of PF in the enucleation group, although the severity of PF tends to be less than in the group undergoing formal resection (73,77). One of the prime arguments against enucleation lies in difficulty with fully evaluating the regional lymph nodes, particularly if curative resection is the goal. Therefore, enucleation should be limited to patients with insulinomas which tend to be on the benign end of the pNET spectrum.
Small pNETs that lie in the pancreatic neck or body and are close to the pancreatic duct may be amenable to a central pancreatectomy. This procedure has the advantage of better pancreatic function, retained gastrointestinal continuity, and preservation of the spleen. Studies comparing central pancreatectomy to standard distal pancreatectomy or pancreaticoduodenectomy in low-grade malignant tumors demonstrate benefits similar to enucleation, including improved blood loss, operative time, and pancreatic exocrine/endocrine function favoring the central pancreatectomy group (78-81). Mortality is similar between the groups. Central pancreatectomy does have a higher rate of PF formation, although these tend to be less severe than those in the standard resection group (78-81). This procedure shares the oncologic concerns surrounding enucleation, including inadequate lymph node sampling, and is only appropriate for select patients who may have been enucleated if not for the location of the neoplasm being deep in the pancreas parenchyma.
Complete oncologic resections for pNET include distal pancreatectomy with or without splenectomy, and pancreaticoduodenectomy. Distal pancreatectomy is indicated for lesions in the body or tail of the pancreas. Spleen-preserving distal pancreatectomy is generally reserved for insulinomas, as these are generally more benign, although there is no clear consensus regarding when preservation of the spleen may be appropriate (82,83). In general, splenectomy is included in distal pancreatic resections of non-insulinoma pNETs to ensure adequate lymph node harvest. Pancreaticoduodenectomy is the standard of care for pNETs found in the head of the pancreas. Rarely, patients with lesions throughout the pancreas may benefit from a total pancreatectomy. Historically, this procedure has been discouraged due to associated morbidity; however, advances in post-operative management have made this procedure a viable option (84-86).
Laparoscopic pancreatic resections
Traditionally, pancreatic resections have been performed as open procedures; however, laparoscopic approaches to each of the aforementioned procedures have been described and are gaining traction. Laparoscopic enucleation of pNETs has only been reported in small series. However, it is reported that operative times and blood loss have been improved in the laparoscopic group compared to open while the rate of PF is similar (87,88). Laparoscopic central pancreatectomy is the least developed of the laparoscopic approaches, but preliminary results indicate its feasibility (89,90). The most widely studied laparoscopic pancreatic resection is distal pancreatectomy, where laparoscopy has clearly been shown to be superior to an open approach in appropriate patients. A large meta-analysis of 18 studies comparing laparoscopic and open distal pancreatectomies found that the laparoscopic group had less blood loss, shorter hospital stays, fewer overall complications, and fewer surgical site infections. There was no difference in operative time, margin positivity, rate of PF, or overall mortality between the two groups (91).
Laparoscopic pancreaticoduodenectomy is still in its infancy but is increasingly being performed at major pancreatic centers. A report of a single center experience comparing laparoscopic and open pancreaticoduodenectomy found improvements in blood loss, decreased use of blood transfusions, and shorter hospital/ICU stays favoring the laparoscopic group. Operative time was significantly longer in the laparoscopic group but there was no difference in the rate of overall complications, PF, or delayed gastric emptying between the two groups. From an oncologic standpoint, there was no difference in margin positivity, tumor size, or TNM staging; in fact there was an improvement in the number of lymph nodes harvested favoring the laparoscopic group (92). An investigation of cost analysis out of the same institution found that, although operating room costs were higher in the laparoscopic group, these costs were recouped in lower subsequent treatment costs and overall cost of care was similar between open and laparoscopic groups (93). It is important to note, however, that laparoscopic approaches to pancreatic resections of all types are still relatively novel and long-term survival and outcomes have yet to be determined. Additionally, laparoscopic approaches to some small tumors, where direct palpation of the lesion is not possible, would be impractical without the contribution of endoscopic tattooing. Use of EUS tattooing has been shown to facilitate tumor identification and reduce operative times during laparoscopic distal pancreatectomy (94). Currently, at our institution, we routinely offer a laparoscopic resection for patients with pNETs regardless of the type of operation required.
The role of surgery in MEN1
In the setting of MEN1, the overall strategy of surgical therapy differs from that of sporadic tumors. Patients with MEN1 tend to present with pNETs at an earlier age and present with multiple tumors (up to 80% of patients) (95). Often these patients will present with multiple small tumors (<0.5 cm), and ultrasound (EUS vs. intra-operative) can be important during workup and treatment. Patients with functional non-gastrinoma pNETs, particularly insulinomas, usually undergo resection due to symptoms of hormone excess. However, surgical resection of small gastrinomas and non-functional pNETs in MEN1 is controversial. Large surgical series with randomized data of these patients are lacking and there are no widely accepted treatment guidelines (95-97). Size criteria for surgical resection range from 1-3 cm, with some arguing for resection of any known tumor (15).
There are several arguments in favor of observation of small gastrinomas and non-functional pNETs. First, pancreatic resection has significant morbidity and mortality. Second, that given the underlying biology of the patient, resection is rarely curative and most patients require re-operation. Third, that although metastatic pNETs are the leading cause of MEN1 related death, this accounts for only about 15% of mortality in MEN1 patients. Lastly, that the survival of these patient, even with metastatic disease, is generally excellent (roughly 50% 15-year survival rate for metastatic gastrinoma) (15,95,98,99). In a prospective series of 81 patients with MEN1 and gastrinoma, patients with small tumors <2.5 cm in size who did not undergo surgical resection had equivalent 15-year survival to MEN1 patients without a pNET (90-100%). Furthermore, patients with tumors between 2.5 and 6 cm who underwent resection had equivalent 15-year survival to patients with small/no tumors, suggesting that salvage surgery for enlarging tumors is possible with little increased risk (99). A retrospective series of 65 patients with MEN1 and non-functioning pNETs <2 cm in size showed no difference in survival between patients who underwent surgical removal of the tumor versus no surgery. Additionally, there was no difference in life-expectancy between patients with <2 cm tumors and those with no pNETs (95). Another retrospective series of 108 patients with MEN1 and non-functional pNETs showed equivalent survival between patients who underwent curative surgery (mean tumor size 3 cm) versus no surgery (mean tumor size 1.6 cm). In this series, patients who developed metastatic disease had poor long term survival of 34% at 8 years (15).
Those in favor of aggressive resection of even small pNETs in MEN1 argue that developing liver metastases significantly shortens life expectancy and remains one of the worst prognostic signs in these patients. They argue that up to 33% of patients with tumors <1 cm already have metastatic disease, and that early resection is the best chance to prevent development of metastases (100). This controversy likely stems from the fact that, while many pNETs in MEN1 remain stable for years, some (about 20-30%) will have aggressive disease (101). Without reliable criteria to prospectively identify aggressive disease, consensus on the indications for surgery in MEN1 patients is unlikely.
The extent of pancreatectomy can also be somewhat controversial. Given the multifocal nature of tumor development in these patients, total pancreatectomy is often necessary to remove all disease, although this is rarely performed. The most common operation is a distal pancreatectomy with enulceation of any tumors in the head of the pancreas. Given the high chance of metastasis to the lymph nodes, the spleen is generally not preserved to ensure an adequate lymph node harvest. Gastrinomas also often occur in the duodenum, and can be very small. Intraoperative endoscopic transillumination, in addition to intraoperative ultrasound and palpation, can aid in the location of these tumors. Some may be removed by duodenotomy and some by pancreas-preserving duodenectomy. Others will require a formal panctreaticoduodenectomy (15,96-98).
Surgical treatment of advanced disease
Cytoreductive surgery
The role of cytoreductive surgery in metastatic pNET is unclear; however most consensus guidelines agree that aggressive resection of the primary tumor, regional lymph nodes, and liver/distant metastases should be pursued if greater than 90% of the tumor burden can be resected (102-106). The rationale behind this approach is that most pNETs have a relatively indolent course compared to other pancreatic neoplasms, and that tumor debulking, while not curative, provides the theoretical advantages of symptom control in functional tumors and prolonged survival in both functional and non-functional pNETs. While the available evidence is not conclusive, it strongly suggests that these premises are correct. A retrospective review of 170 NETs who underwent palliative debulking found that 96% of those with symptoms had resolution post-operatively, and reported a 5- and 10-year survival rate 61% and 35% (107). This is a great improvement over a 5-year survival rate of 30-40% for patients with untreated liver metastases (108,109).
Most recently, a retrospective review of metastatic non-functional pNETs compared patients who underwent R0 versus R1 (>90% removal of tumor burden) resections. Not only did it demonstrate a survival benefit to R1 resections with a 59.9% and 45.5% 5- and 10-year survival rate, but it also found no significant difference in survival rates between the two groups. Of note, patients with R1 progressions did demonstrate a slightly greater propensity towards disease progression with a 3.5% progression free survival rate at five years versus a 10% recurrence free survival rate at five years in the R0 group (102). If greater than 90% of the tumor burden cannot be resected, palliative surgery is not indicated because there is no difference in survival between patients that receive sub-optimal debulking compared to patients that do not undergo surgery (110-112). Furthermore, in patients with un-resectable liver metastases, removal of the primary tumor did not improve survival compared to no surgical intervention (113). Unfortunately, only 5-15% of patients with metastases present with disease appropriate for debulking according to these criteria (103,112).
Treatment of liver metastases
Several treatment options exist to address liver metastases. Partial hepatectomy is possible in many patients while preserving adequate liver function. Locally ablative therapies, such as radiofrequency ablation (RFA), microwave ablation, cryotherapy, or ethanol injection are also options in metastases not amenable to resection. Other possibilities, including transcatheter embolization/transcatheter chemoembolization (TAE/TACE) or radioembolization, are increasingly being utilized. Finally, in select patients with wide spread hepatic metastases, liver transplant may be considered.
Partial hepatectomy
In patients with a primary pNET and synchronous hepatic metastases, hepatectomy can be accomplished in a combined procedure with acceptable morbidity and mortality (102,114). If staged procedures are required, liver resection should be performed first, followed by pancreatic resection, due to the risk of seeding the biliary tract during a prior biliary-enteric diversion. This is particularly true following pancreaticoduodenectomy. In a dual-center series of staged pancreatic/hepatic resections, patients that underwent pancreaticoduodenectomy prior to resection of their liver metastases had a higher risk of forming a hepatic abscess compared to patients who underwent liver resection first (115).
Local ablation
Local ablative therapies include RFA, cryotherapy, microwave coagulation, and ethanol injection, although RFA is the most popular and widely studied. These therapies can be performed percutaneously or during surgery via laparotomy or laparoscopy, and have been shown to complement resection of the primary tumor and amenable liver metastases; making palliative surgery possible for patients that would otherwise not meet criteria (116). Morbidity is low from this procedure with complication rates of 5-15%, usually hematomas or abscesses. Successful local control of liver tumors occurs 85-95% of the time, symptom improvement from hormone excess occurs roughly 90% of the time, and patients can remain without progression of disease for years (117-119). In the largest case series a 5-year survival of 48% was reported which is comparable to surgical metastectomy (117). One advantage of this procedure is that it can be applied multiple times as new metastases emerge.
TAE/TACE
TAE can be employed as palliative therapy in patients with liver metastases not amenable to surgical resection or ablation. It relies on the principle that metastatic tumor cells derive the majority of their oxygen supply from the hepatic artery as opposed to hepatocytes, which receive oxygen primarily from the portal vein. Performed via angiography, embolization may be performed alone (bland embolization) or in combination with chemotherapeutic agents (chemoembolization) (8,40). Multiple chemotherapeutic agents have been tried, including 5-fluorouracil (5-FU), cisplatin, streptozosyn, and, more recently, anthracyclines, such as doxorubicin or epirobicin (8,40,120). No comparison between the various agents exists, and choice of agent is largely empiric. Tumor response rates, derived mainly from retrospective reviews, range from 35-80% in pNETs (120-125). These series also report a symptomatic response of 70-100%, median progression free survival of 10-30 months, and overall survival of 20-36 months. There appears to be no difference in efficacy between TAE and TACE, although there was a trend towards improved response and survival in one series favoring the TACE arm that did not reach clinical significance (124). Another, more recent, series showed equivalent survival and response rates, but significantly increased development of post-embolization syndrome in the TACE group (120). TAE and TACE have never been compared in a randomized trial. Absolute contraindications to the procedure include portal vein thrombosis, ascites, or liver failure. Involvement of more than 50% of the liver by metastases is a relative contraindication given the concern for inducing acute liver failure (8,40,112).
Radioembolization
Radioembolization (RE) is the selective distribution of radioactive yttrium-90 microspheres into the peri-tumoral vasculature via branches of the hepatic artery. Yttrium-90 is a high-energy beta-emitter that has a mean tissue penetrance of 2.5 mm (126,127). Unlike TAE/TACE, vessel occlusion and tissue ischemia are not the goal as radiotherapy has its optimal effect under normal oxygen tensions (128). Because of this, RE is more relatively sparing of normal hepatic tissue than TAE/TACE and can be used on patients with relatively more involvement of the liver by metastases. RE has been better studied in other cancer metastases, with only a few series investigating its use in NETs (129-132). The largest of these is a retrospective review of 148 patients undergoing 185 RE procedures. The study assessed tumor response with imaging and reported that tumor response was stable in 27% of patients, partial in 60.5%, complete in 2.7%, and progression of disease in 4.9%. Median survival was 70 months (132). While most series report a low rate of serious complications with this procedure, serious complications can include symptoms of acute hormone release, acute liver failure, tumor lysis syndrome, post-embolization syndrome, and radiation pneumonitis (126).
Liver transplant
Although usually reserved for patients with life-threatening hormonal imbalances refractory to medical management or diffuse non-functional metastases not responsive to other therapies, orthotopic or living donor liver transplantation has been shown to be successful at alleviating symptoms of hormone excess and improving survival in a select group of patients. A meta-analysis of 103 patients undergoing liver transplant for metastatic NETs reported an overall and disease free 5-year survival of 47% and 24% respectively (133). More recent, smaller series have reported higher survival, ranging from 67-80%, however the rate of cure remains low with disease-free survival ranging from 20-48% at 5 years (134-136). Proposed criteria to optimize outcomes from liver transplantation include age less than 50 years, Ki-67 index of less than 2%, tumors that stain for epithelial cadherin, and lack of extra-hepatic metastases (133-137). Given the limited availability of donors and the currently low cure rates, further efforts to refine selection criteria are essential.
Medical therapy
While the primary treatment for pNETs is surgical, many patients present with advanced disease and are not candidates for resection. Many therapeutic modalities exist for medical management of advanced disease including treatment of the symptoms of excess hormone production, somatostatin analogs, peptide receptor radiotherapy (PRRT), and cytotoxic chemotherapy. As mentioned previously, new information from exome sequencing has made targeted molecular therapies for pNETs possible for the first time.
Managing hormone excess
In patients with unresectable, metastatic, functional pNETs, medical therapy should focus first on control of the hormone excess state, as untreated hormone excess can be a cause of significant morbidity and mortality (8,112). The acid hyper-secretion of ZE syndrome is usually well controlled on proton pump inhibitors. Symptoms of hypoglycemia from an insulinoma can be controlled with small frequent meals and the drug diazoxide, which suppresses insulin secretion. The hyper-hormonal states of other functional tumors can be treated with short- and long-acting somatostatin analogs (8,112).
Somatostatin analogs
Somatostatin analog (SSA) therapy, usually with octreotide or lantreotide, targets somatostatin receptors that are overexpressed on most pNETs. SSAs can be effective at controlling hormone excess in patients with functional tumors; however, they should be used cautiously in patients with insulinomas. Since insulinomas rarely express somatostatin receptors, SSAs can blunt a compensatory glucagon response and exacerbate hypoglycemia. SRS imaging modalities can be useful to evaluate if a particular pNET expresses somatostatin receptors and is a candidate for SSA therapy (8,138). SSAs also seem to have cytostatic effects that can stabilize metastatic disease without tumor regression in most cases. SSAs can impede hormone release and slow cell growth by biding to somatostain receptors, but they also have indirect effects, including inducing apoptosis, suppressing release of growth factors, and inhibiting angiogenesis. The effect of these agents is most pronounced in tumors with a low proliferative index as they have a higher burden of somatostatin receptors (8,139). Investigation of the disease stabilizing effects of SSAs in pNETs has yet to be performed in a prospective manner. However, in a prospective, randomized, controlled study of 85 patients with metastatic mid-gut NETs, long-acting octreotide lengthened median time to tumor progression from 6 months in the placebo group to 14.3 months in the treatment group. There was no significant survival benefit to octreotide, likely due to the small sample size (140). SSAs have long been the workhorse in medical NET therapy, but combination with newer targeted therapeutic agents may further improve outcomes. Combination of long-acting SSAs with the mTOR inhibitor evorolimus and the antiangiogenic monoclonal antibody bevacizumab has shown promise, and multiple trials are ongoing (141-143).
Peptide receptor radiotherapy
PRRT is performed by coupling radioactive isotopes to SSAs, which enables selective delivery of radiotherapy to tumor cells. PRRT is a novel therapy and investigations regarding its efficacy are ongoing. One series of 504 patients with gastroenteropancreatic NETs treated with Lu-177 labeled PRRT reported complete and partial tumor response in 2% and 28% of patients respectively. Median time to disease progression was 40 months, and median overall survival was 48 months, demonstrating an improvement over historical controls. Serious treatment toxicity was observed in 3.6% of patients (144). PRRT is usually performed with a single radioisotope; however, some recent data suggests that treatment with a combination of radioisotopes may be superior. A prospective study of 486 patients with NETs treated with Y-90 labeled SSAs versus alternating cycles of Y-90 labeled SSA with Lu-177 labeled SSAs showed a significant improvement in median survival from 3.96 years in the single radioisotope group to 5.51 years in the dual radioisotope group. Rates of toxicity were similar between the two groups (145). While gaining acceptance in Europe, PRRT is still considered investigational in the United States. PRRT is generally reserved for patients demonstrating progression of liver metastases; however, response to therapy is better in patients with limited liver involvement, suggesting that earlier treatment may be superior. Additionally, PRRT has been suggested as neoadjuvant therapy, enabling surgical resection in two out of six patients in a small trial (146).
Chemotherapy
While only minimally effective in other NETs, pNETs demonstrate a relative sensitivity to chemotherapy. The type of regimen employed depends on the grade of the tumor, with grade 3 (G3) poorly differentiated tumors responding very differently than grade 1 or 2 (G1/G2) well differentiated tumors. The recommended chemotherapeutic regimen for G3 tumors is cisplatin and etoposide. This has been demonstrated to have a 40-70% response rate, however, the duration of response is generally short (103,147,148). Historically, streptomycin based regimens have been standard therapy for G1/G2 pNETs. Initial studies showed a greater than 60% response rate to streptomycin combined with either 5-FU or doxorubicin (149,150), and for almost the last 30 years streptomycin has been the only US Food and Drug Administration (FDA) approved chemotherapeutic agent for NETs. However, the toxicity of this regimen was significant; limiting its use. In addition, more recent studies using more standardized criteria to evaluate tumor progression have failed to corroborate these early results and called into question the efficacy of this regimen (151,152). Most recently, a retrospective series of 84 patients treated with streptomycin, 5-FU, and doxorubicin reported a tumor response rate of 39% with a median response duration of 9.3 months. Two-year overall and progression free survival rates were 74% and 41% respectively (153).
Given these findings, alternative chemotherapeutic regimens are desirable, and the oral alkylating agent temozolomide, particularly in combination with capecitabine, has shown promise. The physiologic mechanism underlying this synergy is unclear, but it has been proposed that capecitabine exposure in cells leads to depletion of O6-methylguanine-DNA methyltransferase (MGMT). MGMT is a DNA repair gene that resists the therapeutic effect of temozolomide, and patients with methylation of the MGMT promoter in their tumor cells, leading to low expression of MGMT, show increased susceptibility to temozolimde in a variety of cancers (154-156). In a series of 30 patients treated with temozolomide in combination with capecitabine, 70% of patients demonstrated a radiographic tumor response. Median progression free survival was 18 months in these patients, and the two-year survival rate was 92%. Toxicity was minimal compared to traditional streptomycin regimens (156). A more recent 18 patient series reports similar findings with a 61% percent response rate, median progression free survival of 14 months, and median overall survival of 83 months in patients treated with combination temozolomide and capecitabine (157). The efficacy shown by temozolomide in these smaller trials warrants further evaluation in large-scale studies.
Targeted molecular therapy
As mentioned before, genomic exome sequencing has defined the most common mutations found in pNETs: MEN1 in 44% of tumors, DAXX in 25% of tumors, ATRX in 18% of tumors, and mTOR pathway genes in 16% of tumors. As aberrant mTOR pathway genes have been found in 16% of pNETs, it is expected, then, that inhibiting mTOR signaling would inhibit tumor growth in at least a subset of patients. Everolimus, an oral mTOR signaling inhibitor, has shown some effect in treating pNETs. Having shown anti-tumor effect in phase II trials (158), a phase III trial was conducted involving 410 patients with G1/G2 pNETs that showed radiologic progression within the last 12 months randomized to receive either everolimus or placebo. Median progression free survival in the everolimus group was 11 months compared to 4.6 months in the placebo group (159). Based on this study, everolimus became the first drug in almost 30 years to be approved by the FDA for treatment of locally advanced, unresectable, or metastatic pNET. Everolimus may be more effective in combination with other therapies, including SSAs as previously mentioned, and more work is required to define its optimal role in pNET therapy. Given that only 16% of pNETs have mutations in mTOR pathway genes, one intriguing possibility for future therapy would be to sequence individual patient tumors and prioritize patients with known mTOR mutations to everolimus therapy (138).
Sunitinib is the other major molecularly targeted therapy under investigation. pNETs are highly vascular, and vascular endothelial growth factor (VEGF) plays a critical role in their development (160). Sunitinib is an oral tyrosine kinase inhibitor that is known to target VEGF receptors. A recent phase III trial randomized 171 patients with advanced well differentiated pNETs to therapy with sunitinib versus placebo. The trial was terminated early when interim analysis showed a difference in progression free survival favoring sunitinib. Median progression free survival was 11.4 months in the sunitinib group with a tumor response rate of 9.3% compared to 5.5 months and 0% in the placebo group (161). Based on these data, sunitinib was also recently approved by the FDA as first line therapy in advanced pNETs.
Conclusions
pNETs are relatively rare tumors comprising 1-2% of all pancreatic neoplasms. They may arise sporadically or as part of an underlying genetic syndrome, most commonly MEN1. The majority of pNETs are non-functional, although some patients will present with symptoms secondary to excess hormone production from a functional tumor. Localization and staging of pNETs are essential to correct management. The cornerstone of therapy for localized disease is surgical resection and laparoscopic approaches to complex pancreatic resections are becoming more commonplace. However, most patients present with metastatic disease and will require a multidisciplinary therapeutic approach. Cytoreductive surgery is generally indicated if greater than 90 percent of the tumor burden can be removed. Liver metastases are common, and a variety of liver directed therapies exist to aid management. Recent advances in our understanding of the molecular pathogenesis of these tumors are making targeted molecular therapy a possibility for the first time, improving the survival of patients with metastatic disease and an otherwise bleak prognosis.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Fraenkel M, Kim MK, Faggiano A, et al. Epidemiology of gastroenteropancreatic neuroendocrine tumours. Best Pract Res Clin Gastroenterol 2012;26:691-703. [PubMed]
- Lawrence B, Gustafsson BI, Chan A, et al. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am 2011;40:1-18. [PubMed]
- Halfdanarson TR, Rubin J, Farnell MB, et al. Pancreatic endocrine neoplasms: epidemiology and prognosis of pancreatic endocrine tumors. Endocr Relat Cancer 2008;15:409-27. [PubMed]
- Grimelius L, Hultquist GT, Stenkvist B. Cytological differentiation of asymptomaticpancreatic islet cell tumours in autopsy material. Virchows Arch A Pathol Anat Histol 1975;365:275-88. [PubMed]
- Kimura W, Kuroda A, Morioka Y. Clinical pathology of endocrine tumors of the pancreas: Analysis of autopsy cases. Dig Dis Sci 1991;36:933-42. [PubMed]
- Halfdanarson TR, Rabe KG, Rubin J, et al. Pancreatic neuroendocrine tumors (PNETs): incidence, prognosis and recent trend toward improved survival. Ann Oncol 2008;19:1727-33. [PubMed]
- Yao JC, Eisner MP, Leary C, et al. Population-based study of islet cell carcinoma. Ann Surg Oncol 2007;14:3492-500. [PubMed]
- Metz DC, Jensen RT. Gastrointestinal neuroendocrine tumors: pancreatic endocrine tumors. Gastroenterology 2008;135:1469-92. [PubMed]
- Oberg K. Pancreatic endocrine tumors. Semin Oncol 2010;37:594-618. [PubMed]
- Lawrence B, Gustafsson BI, Chan A, et al. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am 2011;40:1-18. [PubMed]
- de Wilde RF, Edil BH, Hruban RH, et al. Well-differentiated pancreatic neuroendocrine tumors: from genetics to therapy. Nat Rev Gastroenterol Hepatol 2012;9:199-208. [PubMed]
- Perren A, Anlauf M, Henopp T, et al. Multiple endocrine neoplasia type 1 (MEN1): loss of one MEN1 allele in tumors and monohormonal endocrine cell clusters but not in islet hyperplasia of the pancreas. J Clin Endocrinol Metab 2007;92:1118-28. [PubMed]
- Jensen RT, Berna MJ, Bingham DB, et al. Inherited pancreatic endocrine tumor syndromes: advances in molecular pathogenesis, diagnosis, management, and controversies. Cancer 2008;113:1807-43. [PubMed]
- Pipeleers-Marichal M, Somers G, Willems G, et al. Gastrinomas in the duodenums of patients with multiple endocrine neoplasia type 1 and the Zollinger-Ellison syndrome. N Engl J Med 1990;322:723-7. [PubMed]
- Triponez F, Dosseh D, Goudet P, et al. Epidemiology data on 108 MEN 1 patients from the GTE with isolated nonfunctioning tumors of the pancreas. Ann Surg 2006;243:265-72. [PubMed]
- Carty SE, Helm AK, Amico JA, et al. The variable penetrance and spectrum of manifestations of multiple endocrine neoplasia type 1. Surgery 1998;124:1106-13. [PubMed]
- Alexakis N, Connor S, Ghaneh P, et al. Hereditary pancreatic endocrine tumours. Pancreatology 2004;4:417-33; discussion 434-5. [PubMed]
- Dean PG, van Heerden JA, Farley DR, et al. Are patients with multiple endocrine neoplasia type I prone to premature death? World J Surg 2000;24:1437-41. [PubMed]
- Pellegata NS. MENX and MEN4. Clinics (Sao Paulo) 2012;67 Suppl 1:13-8. [PubMed]
- Thakker RV. Multiple endocrine neoplasia type 1 (MEN1) and type 4 (MEN4). Mol Cell Endocrinol 2014;386:2-15. [PubMed]
- Vortmeyer AO, Huang S, Lubensky I, et al. Nonislet origin of pancreatic islet cell tumors. J Clin Endocrinol Metab 2004;89:1934-8. [PubMed]
- Jiao Y, Shi C, Edil BH, et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science 2011;331:1199-203. [PubMed]
- Heaphy CM, de Wilde RF, Jiao Y, et al. Altered telomeres in tumors with ATRX and DAXX mutations. Science 2011;333:425. [PubMed]
- de Wilde RF, Heaphy CM, Maitra A, et al. Loss of ATRX or DAXX expression and concomitant acquisition of the alternative lengthening of telomeres phenotype are late events in a small subset of MEN-1 syndrome pancreatic neuroendocrine tumors. Mod Pathol 2012;25:1033-9. [PubMed]
- Marinoni I, Kurrer AS, Vassella E, et al. Loss of DAXX and ATRX are associated with chromosome instability and reduced survival of patients with pancreatic neuroendocrine tumors. Gastroenterology 2014;146:453-60. [PubMed]
- Dogeas E, Karagkounis G, Heaphy CM, et al. Alternative lengthening of telomeres predicts site of origin in neuroendocrine tumor liver metastases. J Am Coll Surg 2014;218:628-35. [PubMed]
- Klimsta DS, Armold R, Capella C, et al. Neuroendocrine neoplasms of the pancreas. In: Bosman F, Carneiro F, Hruban RH, et al. eds. WHO Classification of Tumours of the Digestive System. Lyon: IARC Press, 2010:322-6.
- Rindi G, Kloppel G, Alhman H, et al. TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch 2006;449:395-401. [PubMed]
- Exocrine and Endocrine Pancreas. AJCC Cancer Staging Manual. New York, NY: Springer, 2010:241-9.
- Ellison TA, Wolfgang CL, Shi C, et al. A single institution’s 26-year experience with nonfunctional pancreatic neuroendocrine tumors: a validation of current staging systems and a new prognostic nomogram. Ann Surg 2014;259:204-12. [PubMed]
- Strosberg JR, Cheema A, Weber JM, et al. Relapse-free survival in patients with nonmetastatic, surgically resected pancreatic neuroendocrine tumors: an analysis of the AJCC and ENETS staging classifications. Ann Surg 2012;256:321-5. [PubMed]
- Liu TC, Hamilton N, Hawkins W, et al. Comparison of WHO Classifications (2004, 2010), the Hochwald grading system, and AJCC and ENETS staging systems in predicting prognosis in locoregional well-differentiated pancreatic neuroendocrine tumors. Am J Surg Pathol 2013;37:853-9. [PubMed]
- Rindi G, Falconi M, Klersy C, et al. TNM staging of neoplasms of the endocrine pancreas: results from a large international cohort study. J Natl Cancer Inst 2012;104:764-77. [PubMed]
- van Beek AP, de Haas ER, van Vloten WA, et al. The glucagonoma syndrome and necrolytic migratory erythema: a clinical review. Eur J Endocrinol 2004;151:531-7. [PubMed]
- Garbrecht N, Anlauf M, Schmitt A, et al. Somatostatin-producing neuroendocrine tumors of the duodenum and pancreas: incidence, types, biological behavior, association with inherited syndromes, and functional activity. Endocr Relat Cancer 2008;15:229-41. [PubMed]
- Grant CS. Insulinoma. Best Pract Res Clin Gastroenterol 2005;19:783-98. [PubMed]
- Berna MJ, Hoffmann KM, Serrano J, et al. Serum gastrin in Zollinger-Ellison syndrome: I. Prospective study of fasting serum gastrin in 309 patients from the National Institutes of Health and comparison with 2229 cases from the literature. Medicine (Baltimore) 2006;85:295-330. [PubMed]
- Ruszniewski P, Podevin P, Cadiot G, et al. Clinical, anatomical, and evolutive features of patients with the Zollinger-Ellison syndrome combined with type I multiple endocrine neoplasia. Pancreas 1993;8:295-304. [PubMed]
- Berna MJ, Hoffmann KM, Long SH, et al. Serum gastrin in Zollinger-Ellison syndrome: II. Prospective study of gastrin provocative testing in 293 patients from the National Institutes of Health and comparison with 537 cases from the literature: evaluation of diagnostic criteria, proposal of new criteria, and correlations with clinical and tumoral features. Medicine (Baltimore) 2006;85:331-64. [PubMed]
- Milan SA, Yeo CJ. Neuroendocrine tumors of the pancreas. Curr Opin Oncol 2012;24:46-55. [PubMed]
- Mullans EA, Cohen PR. Iatrogenic necrolytic migratory erythema: a case report and review of nonglucagonoma-associated necrolytic migratory erythema. J Am Acad Dermatol 1998;38:866-73. [PubMed]
- Appetecchia M, Ferretti E, Carducci M, et al. Malignant glucagonoma. New options of treatment. J Exp Clin Cancer Res 2006;25:135-9. [PubMed]
- Nikou GC, Toubanakis C, Nikolaou P, et al. VIPomas: an update in diagnosis and management in a series of 11 patients. Hepatogastroenterology 2005;52:1259-65. [PubMed]
- Ghaferi AA, Chojnacki KA, Long WD, et al. Pancreatic VIPomas: subject review and one institutional experience. J Gastrointest Surg 2008;12:382-93. [PubMed]
- Paik WH, Ryu JK, Song BJ, et al. Clinical usefulness of plasma chromogranin A in pancreatic neuroendocrine neoplasm. J Korean Med Sci 2013;28:750-4. [PubMed]
- Singh S, Law C. Chromogranin A: a sensitive biomarker for the detection and post-treatment monitoring of gastroenteropancreatic neuroendocrine tumors. Expert Rev Gastroenterol Hepatol 2012;6:313-34. [PubMed]
- Vinik AI, Silva MP, Woltering EA, et al. Biochemical testing for neuroendocrine tumors. Pancreas 2009;38:876-89. [PubMed]
- Bajetta E, Ferrari L, Martinetti A, et al. Chromogranin A, neuron specific enolase, carcinoembryonic antigen, and hydroxyindole acetic acid evaluation in patients with neuroendocrine tumors. Cancer 1999;86:858-65. [PubMed]
- Oberg K. Circulating biomarkers in gastroenteropancreatic neuroendocrine tumours. Endocr Relat Cancer 2011;18 Suppl 1:S17-25. [PubMed]
- Anders S. Radiological and nuclear medicine imaging of gastroenteropancreatic neuroendocrine tumors. Best Pract Res Clin Gastroentero 2012;26:803-18.
- Herwick S, Miller FH, Keppke AL. MRI of islet cell tumors of the pancreas. AJR Am J Roentgenol 2006;187:W472-80. [PubMed]
- Singhi AD, Chu LC, Tatsas AD, et al. Cystic pancreatic neuroendocrine tumors: a clinicopathologic study. Am J Surg Pathol 2012;36:1666-73. [PubMed]
- Sundin A, Vullierme MP, Kaltsas G, et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: radiological examinations. Neuroendocrinology 2009;90:167-83. [PubMed]
- Caramella C, Dromain C, De Baere T, et al. Endocrine pancreatic tumors: which are the most useful MRI sequences? Eur Radiol 2010;20:2618-27. [PubMed]
- Oberg K. Diagnostic work-up of gastroenteropancreatic neuroendocrine tumors. Clinics (Sao. Paulo) 2012;67:109-12. [PubMed]
- Joseph S, Wang YZ, Boudreaux JP, et al. Neuroendocrine tumors: current recommendations for diagnosis and surgical management. Endocrinol Metab Clin North Am 2011;40:205-31. [PubMed]
- de Herder WW, Kwekkeboom DJ, Valkema R, et al. Neuroendocrine tumors and somatostatin: imaging techniques. J Endocrinol Invest 2005;28:132-6. [PubMed]
- Garin E, Le Jeune F, Devillers A, et al. Predictive value of 18F-FDG PET and somatostatin receptor scintigraphy in patients with metastatic endocrine tumors. J Nucl Med 2009;50:858-64. [PubMed]
- Binderup T, Knigge U, Loft A, et al. 18F-fluorodeoxyglucose positron emission tomography predicts survival of patients with neuroendocrine tumors. Clin Cancer Res 2010;16:978-85. [PubMed]
- Gabriel M, Decristoforo C, Kendler D, et al. 68 Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: comparison with somatostatin receptor scintigraphy and CT. J Nucl Med 2007;48:508-18. [PubMed]
- Buchmann I, Henze M, Engelbrecht S, et al. Comparison of 68Ga-DOTATOC PET and 111In-DTPAOC (OctreoScan) SPECT in patients with neuroendocrine tumours. Eur J Nucl Med Mol Imaging 2007;34:1617-26. [PubMed]
- Srirajaskanthan R, Kayani I, Quigley AM, et al. The role of 68Ga-DOTATATE PET in patients with neuroendocrine tumors and negative or equivocal findings on 111In-DTPA-octreotide scintigraphy. J Nucl Med 2010;51:875-82. [PubMed]
- Frilling A, Sotiropoulos GC, Radtke A, et al. The impact of 68Ga-DOTATOC positron emission tomography/computed tomography on the multimodal management of patients with neuroendocrine tumors. Ann Surg 2010;252:850-6. [PubMed]
- Hofman MS, Kong G, Neels OC, et al. High management impact of Ga-68 DOTATATE (GaTate) PET/CT for imaging neuroendocrine and other somatostatin expressing tumours. J Med Imaging Radiat Oncol 2012;56:40-7. [PubMed]
- Ambrosini V, Campana D, Bodei L, et al. 68Ga-DOTANOC PET/CT clinical impact in patients with neuroendocrine tumors. J Nucl Med 2010;51:669-73. [PubMed]
- Kumar R, Sharma P, Garg P, et al. Role of (68)Ga-DOTATOC PET-CT in the diagnosis and staging of pancreatic neuroendocrine tumours. Eur Radiol 2011;21:2408-16. [PubMed]
- Naswa N, Sharma P, Kumar A, et al. Gallium-68-DOTA-NOC PET/CT of patients with gastroenteropancreatic neuroendocrine tumors: a prospective single-center study. AJR Am J Roentgenol 2011;197:1221-8. [PubMed]
- Frilling A, Sotiropoulos GC, Li J, et al. Multimodal management of neuroendocrine liver metastases. HPB (Oxford) 2010;12:361-79. [PubMed]
- Kim MK. Endoscopic ultrasound in gastroenteropancreatic neuroendocrine tumors. Gut Liver 2012;6:405-10. [PubMed]
- Atiq M, Bhutani MS, Bektas M, et al. EUS-FNA for pancreatic neuroendocrine tumors: a tertiary cancer center experience. Dig Dis Sci 2012;57:791-800. [PubMed]
- Lennon AM, Newman N, Makary MA, et al. EUS-guided tattooing before laparoscopic distal pancreatic resection (with video). Gastrointest Endosc 2010;72:1089-94. [PubMed]
- Cauley CE, Pitt HA, Ziegler KM, et al. Pancreatic enucleation: improved outcomes compared to resection. J. Gastrointest Surg 2012;16:1347-53. [PubMed]
- Pitt SC, Pitt HA, Baker MS, et al. Small pancreatic and periampullary neuroendocrine tumors: resect or enucleate? J Gastrointest Surg 2009;13:1692-8. [PubMed]
- Casadei R, Ricci C, Rega D, et al. Pancreatic endocrine tumors less than 4 cm in diameter: resect or enucleate? A single-center experience. Pancreas 2010;39:825-8. [PubMed]
- Hackert T, Hinz U, Fritz S, et al. Enucleation in pancreatic surgery: indications, technique, and outcome compared to standard pancreatic resections. Langenbecks Arch Surg 2011;396:1197-203. [PubMed]
- Inchauste SM, Lanier BJ, Libutti SK, et al. Rate of clinically significant postoperative pancreatic fistula in pancreatic neuro- endocrine tumors. World J Surg 2012;36:1517-26. [PubMed]
- Cherif R, Gaujoux S, Couvelard A, et al. Parenchyma-sparing resections for pancreatic neuroendocrine tumors. J Gastrointest Surg 2012;16:2045-55. [PubMed]
- Falconi M, Zerbi A, Crippa S, et al. Parenchyma-preserving resections for small nonfunctioning pancreatic endocrine tumors. Ann Surg Oncol 2010;17:1621-27. [PubMed]
- Lavu H, Knuth JL, Baker MS, et al. Middle segment pancreatectomy can be safely incorporated into a pancreatic surgeon’s clinical practice. HPB (Oxford) 2008;10:491-7. [PubMed]
- Du ZY, Chen S, Han BS, et al. Middle segmental pancreatectomy: a safe and organ-preserving option for benign and low-grade malignant lesions. World J Gastroenterol 2013;19:1458-65. [PubMed]
- Shikano T, Nakao A, Kodera Y, et al. Middle pancreatectomy: safety and long-term results. Surgery 2010;147:21-9. [PubMed]
- Shoup M, Brennan MF, McWhite K, et al. The value of splenic preservation with distal pancreatectomy. Arch Surg 2002;137:164-8. [PubMed]
- Kimura W, Tezuka K, Hirai I. Surgical management of pancreatic neuroendocrine tumors. Surg Today 2011;41:1332-43. [PubMed]
- Casadei R, Monari F, Buscemi S, et al. Total pancreatectomy: indications, operative technique, and results: a single centre experience and review of literature. Updates Surg 2010;62:41-6. [PubMed]
- Imamura M. Recent standardization of treatment strategy for pancreatic neuroendocrine tumors. World J Gastroenterol 2010;16:4519-25. [PubMed]
- Heidt DG, Burant C, Simeone DM. Total pancreatectomy: indications, operative technique, and postoperative sequelae. J Gastrointest Surg 2007;11:209-16. [PubMed]
- Crippa S, Boninsegna L, Partelli S, et al. Parenchyma-sparing resections for pancreatic neoplasms. J Hepatobiliary Pancreat Sci 2010;17:782-7. [PubMed]
- Kooby DA, Chu CK. Laparoscopic management of pancreatic malignancies. Surg Clin North Am 2010;90:427-46. [PubMed]
- Kuroki T, Eguchi S. Laparoscopic parenchyma-sparing pancreatectomy. J Hepatobiliary Pancreat Sci 2014;21:323-7. [PubMed]
- Cheng K, Shen B, Peng C, et al. Initial experiences in robot-assisted middle pancreatectomy. HPB 2013;15:315-21. [PubMed]
- Venkat R, Edil BH, Schulick RD, et al. Laparoscopic distal pancreatectomy is associated with significantly less overall morbidity compared to the open technique: a systematic review and meta-analysis. Ann Surg 2012;255:1048-59. [PubMed]
- Asbun HJ, Stauffer JA. Laparoscopic vs open pancreaticoduodenectomy: overall outcomes and severity of complications using the Accordion Severity Grading System. J Am Coll Surg 2012;215:810-9. [PubMed]
- Mesleh MG, Stauffer JA, Bowers SP, et al. Cost analysis of open and laparoscopic pancreaticoduodenectomy: a single institution comparison. Surg Endosc 2013;27:4518-23. [PubMed]
- Newman NA, Lennon AM, Edil BH, et al. Preoperative endoscopic tattooing of pancreatic body and tail lesions decreases operative time for laparoscopic distal pancreatectomy. Surgery 2010;148:371-7. [PubMed]
- Triponez F, Goudet P, Dosseh D, et al. Is surgery beneficial for MEN1 patients with small (<2cm), nonfunctioning, pancreaticoduodenal endocrine tumor? An analysis of 65 patients from the GTE. World J Surg 2006;30:654-62. [PubMed]
- Niina Y, Fujimori N, Nakamura T, et al. The current strategy for managing pancreatic neuroendocrine tumors in multiple endocrine neoplasia type 1. Gut Liver 2012;6:287-94. [PubMed]
- Machado MC. Surgical treatment of pancreatic endocrine tumors in multiple endocrine neoplasia type 1. Clinics (Sao Paulo) 2012;67 Suppl 1:145-8. [PubMed]
- Norton JA, Jensen RT. Resolved and unresolved controversies in the surgical management of patients with Zollinger-Ellison syndrome. Ann Surg 2004;240:757-73. [PubMed]
- Norton JA, Alexander HR, Fraker DL, et al. Comparison of surgical results in patients with advanced and limited disease with multiple endocrine neoplasia type 1 and Zollinger-Ellison syndrome. Ann Surg 2001;234:495-505; discussion 505-6. [PubMed]
- Lowney JK, Frisella MM, Lairmore TC, et al. Pancreatic islet cell tumor metastasis in multiple endocrine neoplasia type 1: correlation with primary tumor size. Surgery 1998;124:1043-8. [PubMed]
- Gibril F, Venzon DJ, Ojeaburu JV, et al. Prospective study of the natural history of gastrinoma in patients with MEN1: definition of an aggressive and a nonaggressive form. J Clin Endocrinol Metab 2001;86:5282-93. [PubMed]
- Cusati D, Zhang L, Harmsen WS, et al. Metastatic nonfunctioning pancreatic neuroendocrine carcinoma to liver: surgical treatment and outcomes. J Am Coll Surg 2012;215:117-24. [PubMed]
- Kulke MH, Anthony LB, Bushnell DL, et al. North American Neuroendocrine Tumor Society (NANETS). NANETS treatment guidelines: well-differentiated neuroendocrine tumors of the stomach and pancreas. Pancreas 2010;39:735-52. [PubMed]
- Norton JA. Endocrine tumours of the gastrointestinal tract. Surgical treatment of neuroendocrine metastases. Best Pract Res Clin Gastroenterol 2005;19:577-83. [PubMed]
- Que FG, Sarmiento JM, Nagorney DM. Hepatic surgery for metastatic gastrointestinal neuroendocrine tumors. Adv Exp Med Biol 2006;574:43-56. [PubMed]
- Falconi M, Bettini R, Boninsegna L, et al. Surgical strategy in the treatment of pancreatic neuroendocrine tumors. JOP 2006;7:150-6. [PubMed]
- Sarmiento JM, Heywood G, Rubin J, et al. Surgical treatment of neuroendocrine metastases to the liver: a plea for resection to increase survival. J Am Coll Surg 2003;197:29-37. [PubMed]
- Thompson GB, van Heerden JA, Grant CS, et al. Islet cell carcinomas of the pancreas: a twenty-year experience. Surgery 1988;104:1011-7. [PubMed]
- Chen H, Hardaacre JM, Uzar A, et al. Isolated liver metastasis from neuroendocrine tumors: does resection prolong survival? J Am Coll Surg 1998;187:88-92. [PubMed]
- Jaeck D, Oussoultzoglou E, Bachellier P, et al. Hepatic metastasis from gastroenteropancreatic neuroendocrine tumors: safe hepatic surgery. World J Surg 2001;25:689-92. [PubMed]
- Chamberlain RS, Canes D, Brown KT, et al. Hepatic neuroendocrine metastases: does intervention alter outcomes? J Am Coll Surg 2000;190:432-45. [PubMed]
- Ito T, Igarashi H, Jensen RT. Therapy of metastatic pancreatic neuroendocrine tumors (pNETs): recent insights and advances. J Gastroenterol 2012;47:941-60. [PubMed]
- Bettini R, Mantovani W, Boninsegna L, et al. Primary tumour resection in metastatic nonfunctioning pancreatic endocrine carcinomas. Dig Liver Dis 2009;41:49-55. [PubMed]
- Gaujoux S, Gonen M, Tang L, et al. Synchronous resection of primary and liver metastases for neuroendocrine tumors. Ann Surg Oncol 2012;19:4270-7. [PubMed]
- De Jong MC, Farnell MB, Sclabas G, et al. Liver-directed therapy for hepatic metastases in patients undergoing pancreaticoduodenectomy: a dual-center analysis. Ann Surg 2010;252:142-8. [PubMed]
- Taner T, Atwell TD, Zhang L, et al. Adjunctive radiofrequency ablation of metastatic neuroendocrine cancer to the liver complements surgical resection. HPB (Oxford) 2013;15:190-5. [PubMed]
- Mazzaglia PJ, Berber E, Milas M, et al. Laparoscopic radiofrequency ablation of neuroendocrine liver metastases: a 10-year experience evaluating predictors of survival. Surgery 2007;142:10-9. [PubMed]
- Gillams A, Cassoni A, Conway G, et al. Radiofrequency ablation of neuroendocrine liver metastases: the Middlesex experience. Abdom Imaging 2005;30:435-41. [PubMed]
- Elvin A, Skogseid B, Hellman P. Radiofrequency ablation of neuroendocrine liver metastases. Abdom Imaging 2005;30:427-34. [PubMed]
- Fiore F, Del Prete M, Franco R, et al. Transarterial embolization (TAE) is equally effective and slightly safer than transarterial chemoembolization (TACE) to manage liver metastases in neuroendocrine tumors. Endocrine 2014;47:177-82. [PubMed]
- Eriksson BK, Larsson EG, Skogseid BM, et al. Liver embolizations of patients with malignant neuroendocrine gastrointestinal tumors. Cancer 1998;83:2293-301. [PubMed]
- Ruszniewski P, Rougier P, Roche A, et al. Hepatic arterial chemoembolization in patients with liver metastases of endocrine tumors. A prospective phase II study in 24 patients. Cancer 1993;71:2624-30. [PubMed]
- Strosberg JR, Choi J, Cantor AB, et al. Selective hepatic artery embolization for treatment of patients with metastatic carcinoid and pancreatic endocrine tumors. Cancer Control 2006;13:72-8. [PubMed]
- Gupta S, Johnson MM, Murthy R, et al. Hepatic arterial embolization and chemoembolization for the treatment of patients with metastatic neuroendocrine tumors: variables affecting response rates and survival. Cancer 2005;104:1590-602. [PubMed]
- Akahori T, Sho M, Tanaka T, et al. Significant efficacy of new transcatheter arterial chemoembolization technique for hepatic metastases of pancreatic neuroendocrine tumors. Anticancer Res 2013;33:3355-8. [PubMed]
- Alistar A, Sung M, Kim M, et al. Clinical pathways for pancreatic neuroendocrine tumors. J Gastrointest Cancer 2012;43:532-40. [PubMed]
- Reidy-Lagunes D, Thornton R. Pancreatic neuroendocrine and carcinoid tumors: what’s new, what’s old, and what’s different? Curr Oncol Rep 2012;14:249-56. [PubMed]
- Dickson PV, Behrman SW. Management of pancreatic neuroendocrine tumors. Surg Clin North Am 2013;93:675-91. [PubMed]
- Kalinowski M, Dressler M, Konig A, et al. Selective internal radiotherapy with Yttrium-90 microspheres for hepatic metastatic neuroendocrine tumors: a prospective single center study. Digestion 2009;79:137Y142.
- Cao CQ, Yan TD, Bester L, et al. Radioembolization with yttrium microspheres for neuroendocrine tumour liver metastases. Br J Surg 2010;97:537-43. [PubMed]
- Rhee TK, Lewandowski RJ, Liu DM, et al. 90Y radioembolization for metastatic neuroendocrine liver tumors: preliminary results from a multi-institutional experience. Ann Surg 2008;247:1029-35. [PubMed]
- Kennedy AS, Dezarn WA, McNeillie P, et al. Radioembolization for unresectable neuroendocrine hepatic metastases using resin 90Y-microspheres: early results in patients. Am J Clin Oncol 2008;31:271-9. [PubMed]
- Lehnert T. Liver transplantation for metastatic neuroendocrine carcinoma: an analysis of 103 patients. Transplantation 1998;66:1307-12. [PubMed]
- Frilling A, Malago M, Weber F, et al. Liver transplantation for patients with metastatic endocrine tumors: single-center experience with 15 patients. Liver Transpl 2006;12:1089-96. [PubMed]
- Olausson M, Friman S, Herlenius G, et al. Orthotopic liver or multivisceral transplantation as treatment of metastatic neuroendocrine tumors. Liver Transpl 2007;13:327-33. [PubMed]
- Rosenau J, Bahr MJ, von Wasielewski R, et al. Ki67, E-cadherin, and p53 as prognostic indicators of long-term outcome after liver transplantation for metastatic neuroendocrine tumors. Transplantation 2002;73:386-94. [PubMed]
- Mazzaferro V, Pulvirenti A, Coppa J. Neuroendocrine tumors metastatic to the liver: how to select patients for liver transplantation? J Hepatol 2007;47:460-6. [PubMed]
- Sadaria MR, Hruban RH, Edil BH. Advancements in pancreatic neuroendocrine tumors. Expert Rev Gastroenterol Hepatol 2013;7:477-90. [PubMed]
- Panzuto F, Di Fonzo M, Iannicelli E, et al. Long-term clinical outcome of somatostatin analogues for treatment of progressive, metastatic, well-differentiated entero-pancreatic endocrine carcinoma. Ann Oncol 2006;17:461-6. [PubMed]
- Rinke A, Muller HH, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol 2009;27:4656-63. [PubMed]
- Yao JC, Phan A, Hoff PM, et al. Targeting vascular endothelial growth factor in advanced carcinoid tumor: a random assignment phase II study of depot octreotide with bevacizumab and pegylated interferon alpha-2b. J Clin Oncol 2008;26:1316-23. [PubMed]
- Pavel ME, Hainsworth JD, Baudin E, et al. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT 2): a randomised, placebocontrolled, phase 3 study. Lancet 2011;378:2005-12. [PubMed]
- Toumpanakis C, Caplin ME. Update on the role of somatostatin analogs for the treatment of patients with gastroenteropancreatic neuroendocrine tumors. Semin Oncol 2013;40:56-68. [PubMed]
- Kwekkeboom DJ, de Herder WW, Kam BL, et al. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3] octreotate: toxicity, efficacy, and survival. J Clin Oncol 2008;26:2124-30. [PubMed]
- Villard L, Romer A, Marincek N, et al. Cohort study of somatostatin-based radiopeptide therapy with [(90)Y-DOTA]-TOC versus [(90)Y-DOTA]-TOC plus [(177)Lu-DOTA]-TOC in neuroendocrine cancers. J Clin Oncol 2012;30:1100-6. [PubMed]
- Sowa-Staszczak A, Pach D, Chrzan R, et al. Peptide receptor radionuclide therapy as a potential tool for neoadjuvant therapy in patients with inoperable neuroendocrine tumours (NETs). Eur J Nucl Med Mol Imaging 2011;38:1669-74. [PubMed]
- Öberg K, Knigge U, Kwekkeboom D, et al. Peptide receptor radionuclide therapy as a potential tool for neoadjuvant therapy in patients with inent and follow-up. Ann Oncol 2012;23 Suppl 7:vii124-30. [PubMed]
- Eriksson B, Annibale B, Bajetta E, et al. Mallorca Consensus Conference participants; European Neuroendocrine Tumor Society. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: chemotherapy in patients with neuroendocrine tumors. Neuroendocrinology 2009;90:214-9. [PubMed]
- Moertel CG, Hanley JA, Johnson LA. Streptozocin alone compared with streptozocin plus fluorouracil in the treatment of advanced islet-cell carcinoma. N Engl J Med 1980;303:1189-94. [PubMed]
- Moertel CG, Lefkopoulo M, Lipsitz S, et al. Streptozocin-doxorubicin, streptozocin-fluorouracil or chlorozotocin in the treatment of advanced islet-cell carcinoma. N Engl J Med 1992;326:519-23. [PubMed]
- Cheng PN, Saltz LB. Failure to confirm major objective antitumor activity for streptozocin and doxorubicin in the treatment of patients with advanced islet cell carcinoma. Cancer 1999;86:944-8. [PubMed]
- McCollum AD, Kulke MH, Ryan DP, et al. Lack of efficacy of streptozocin and doxorubicin in patients with advanced pancreatic endocrine tumors. Am J Clin Oncol 2004;27:485-8. [PubMed]
- Kouvaraki MA, Ajani JA, Hoff P, et al. Fluorouracil, doxorubicin, and streptozocin in the treatment of patients with locally advanced and metastatic pancreatic endocrine carcinomas. J Clin Oncol 2004;22:4762-71. [PubMed]
- Esteller M, Garcia-Foncillas J, Andion E, et al. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med 2000;343:1350-4. [PubMed]
- Hegi ME, Liu L, Herman JG, et al. Correlation of O6-methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. J Clin Oncol 2008;26:4189-99. [PubMed]
- Strosberg JR, Fine RL, Choi J, et al. First-line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer 2011;117:268-75. [PubMed]
- Fine RL, Gulati AP, Krantz BA. Capecitabine and temozolomide (CAPTEM) for metastatic, well-differentiated neuroendocrine cancers: The Pancreas Center at Columbia University experience. Cancer Chemother Pharmacol 2013;71:663-70. [PubMed]
- Yao JC, Phan AT, Chang DZ, et al. Efficacy of RAD001 (everolimus) and octreotide LAR in advanced low- to intermediate-grade neuroendocrine tumors: results of a phase II study. J Clin Oncol 2008;26:4311-8. [PubMed]
- Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 2011;364:514-23. [PubMed]
- Zhang J, Jia Z, Li Q, et al. Elevated expression of vascular endothelial growth factor correlates with increased angiogenesis and decreased progression-free survival among patients with low-grade neuroendocrine tumors. Cancer 2007;109:1478-86. [PubMed]
- Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 2011;364:501-13. [PubMed]


