Endoscopy-assisted breast-conserving surgery for breast cancer patients
Introduction
Breast-conserving surgery (BCS) combined with postoperative radiotherapy is widely accepted as standard therapy for early breast cancer patients with survival equivalent to that of mastectomy (1-3). Moreover, oncoplastic breast cancer surgery techniques have developed over the recent years, and patients’ concern regarding the postoperative aesthetic outcome has also been increasing. Therefore, the current goal of BCS is not only to achieve a curative partial resection but also to preserve the cosmetic appearance of the breast. Endoscopy-assisted breast conserving-surgery (EBCS), which has the advantage of a less noticeable scar, was developed more than ten years ago and performed in some Asian countries (4-13). Although a shortage of clinical studies exists, achievement of oncological safety, as well as better cosmetic outcomes, with EBCS has been demonstrated in some institutions recently. However, achieving both tasks (curative operation and satisfaction with the aesthetic outcome) together with EBCS can be challenging and sometimes result in failure because of difficulty in repairing the excised breast volume. In this article, we will review the EBCS clinical studies that have been conducted so far and discuss current issues regarding this operative method.
Overview of the studies
A literature search was performed using the PubMed/Medline database. The following MeSH headings were used: “Endoscopy assisted”, “Breast Cancer”, “Breast Surgery”, “Breast-Conserving Surgery”, “Endoscope” and “Quadrantectomy”. We reviewed six case series and four cohort studies published between December 2001 and August 2013 as representative EBCS studies. The following items will be discussed in this review:
- Indication and contraindication;
- Preoperative diagnostic imaging and marking;
- Operative method;
- Surgical outcomes;
- Oncological outcomes;
- Aesthetic estimation and patient satisfaction rating.
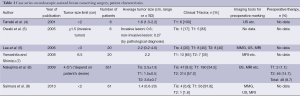
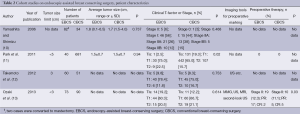
Indications and contraindications for EBCS (Tables 1,2)
Full table
Full table
As for the clinical T-factor, all studies limited their indication to T1 or T2 tumors (average tumor size, 0.6-2.2 cm), and patients who had invasion to the skin, pectoralis muscle, or chest wall were contraindicated. In addition, multicentricity seemed to be contraindicated, similar to that of conventional breast-conserving surgery (CBCS) (6). One study limited resection of the mammary gland region to less than 20% of the total area (13). Clinically positive axillary nodes were also contraindicated in five studies (4,6,9-11).
Preoperative diagnostic imaging and marking (Tables 1,2)
For preoperative diagnosis or marking, five studies used not only mammography (MMG) and ultrasonography (US), but also magnetic resonance imaging (MRI) (6-9,13). Three studies did not mention any diagnostic imaging tools. One study demonstrated a method for preoperative marking using US-guided planning of the resection range and mobilization range of the mammary gland according to the MRI findings. For nonpalpable tumors with calcification identified using MMG, a hook wire was initially inserted into the calcification by stereo-guided insertion. After this, US-guided planning was performed. During marking, patients were laid in an operative position. The surgical margin was marked 1.5-2 cm from the tumor edge. The authors of this study also marked the penetrating branch of the internal thoracic vessels in the parasternal area on the tumor side by US (13).
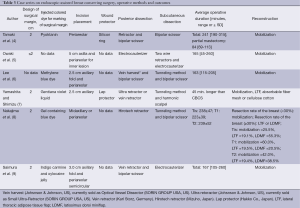
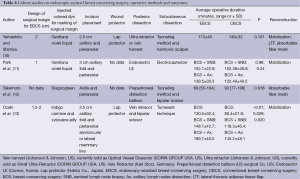
Operative method (Tables 3,4)
Full table
Full table
Setting an intraoperative surgical margin and injection of a colored dye
In five studies, an intraoperative surgical margin was set 2 cm from the tumor border (4,7-10). In other four studies, the margin was set at 1, 1.5-2, and <2 cm (5,11,13), and one study did not demonstrate a surgical margin (6). On the other hand, injection of a colored dye along the resection margin was performed in nine studies with various color dyes, and one study did not discuss the usage of a colored dye.
Skin incision placement
As for the site for a skin incision, axillary, periareolar, midline, and lateral mammary lines were adopted. A combination of an axillary incision with a periareolar incision was commonly used. A small axillary incision (2.5-3 cm) was mainly used for dissection of the posterior surface of the mammary gland in six studies (Figure 1A) (6,7,9-11,13). A periareolar incision was used for the development of a skin flap and the removal of a resected specimen from the breast in nine studies (4-6,8-13). In case of tumors located in the outer upper or outer inner region, a single midaxillary incision or a combination of a small lateral incision (2.5-3 cm) and a small axillary incision was used (8,13). In one study, a single, large axillary incision was used for the partial resection of the mammary gland and for the harvesting of a latissimus dorsi mini-flap (LDF) in order to reconstruct the breast (8).
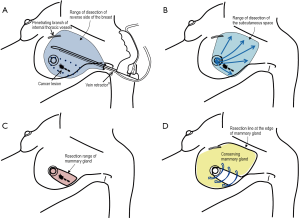
Dissection of the posterior surface of the breast
Endoscopic dissection of the posterior surface was performed with various retraction devices (Vein harvest, Ultra Retractor, Vein Retractor, Endosector LE etc.; Tables 3,4) and a bipolar scissor. These retraction devices allow for a magnified view and the performance of an extensive posterior breast dissection (Figure 1A). In one study, a preperitoneal distention balloon was also effectively used via a periareolar incision (12).
Skin flap development
In five studies, a skin flap was made using a bladeless trocar equipped with an endoscope and bipolar scissors, harmonic scalpels, or an electric cautery (Figure 1B) (6-8,10,12). First, the skin was separated from the mammary gland by a bladeless trocar under video guidance, and multiple tunnels were created. The thickness of the skin can be adjusted by the intensity of the light transmitted from the skin. This method is the so-called “subcutaneous tunneling method”. After that, the septa between the tunnels were dissected using bipolar scissors, harmonic scalpels, or an electric cautery with or without light guidance. Under light- or endoscopic guidance, a skin flap was also made with an electric cauterizer and a bipolar scissor in three studies (5,9,11) and one study (4), respectively. Additionally, in one study, a skin flap was made using subcutaneous hydrodissection and dissecting scissor (13).
Wound protector
To protect the periareolar wound and ensure adequate visualization, a wound protector was used in four studies (4,7,10,13).
Resection of the mammary gland and specimen retrieval
Resection of the mammary gland and specimen retrieval were performed via a periareolar incision (Figure 1C) or midaxillary incision. In one study, a small axillary incision was used not only for resection of the mammary gland, but also for retrieval of the specimen using Endocatch (Autosuture, US) (7).
Placement of surgical clips
To inform the radiation oncologists of accurate surgical margins, surgical clips should be placed in each margin. In two studies, surgical clips were placed in the margins (11,13). The other eight studies did not discuss these data.
Reconstruction of the breast
To repair the defect of the excised breast tissue, volume displacement using the remnant breast tissue, volume replacement using an LDP, and a filling method were used as follows.
Volume displacement
To repair the breast, remnant breast tissue was dissected and mobilized to the defect and sutured via an incision under light guidance (Figure 1D) (4-6,8,9,11,13).
Volume replacement
In one study, if the defect in the breast tissue reached more than 30% of the total breast volume, reconstruction was performed using an LDF harvested under endoscopic guidance or lateral thoracic adipose tissue flap (LTF) via a midaxillary line incision (8).
Filling method
Three studies showed a unique method of filling the breast tissue defect using an absorbent synthetic fiber mesh (Vicryl mesh, Johnson & Johnson Co, US) wrapped in an absorbable adhesion barrier (INTERCEED, Johnson & Johnson Co, US) or oxidized cellulose cotton (Surgicel, Johnson & Johnson Co, US) (7,10,12,14).
Postoperative drainage
Postoperative drainage was routinely performed in four studies (6,10,11,13) and only in case of axillary dissection in two studies (4,9). The other four studies did not discuss these data. As the incision for the drainage tube placement was tiny, it would not seem to affect the aesthetic outcome.
Skin closure and care of the operative scar
The skin incision was closed with a monolayer suture by a buried subcuticular suture or bilayer suture by a buried subcuticular and skin suture or Dermabond (Ethicon Endo-Surgery, US). In one study, after suture removal, the wound was sealed and covered with Micropore (3M Health Care, US) for three months after surgery to keep the wound inconspicuous (13).
Surgical outcomes (Tables 5,6)
Full table
Full table
Operative duration
According to the results of the four cohort studies, an average operative duration for EBCS was equal, 30-50 min longer, or 24 min shorter than that of the CBCS group (10-13). However, these different results can be attributed to the difference in the reconstruction method for each EBCS operative method. Therefore, to compare the operative duration between EBCS and CBCS, standardization of the operative method for EBCS was necessary in the first place.
Intraoperative blood loss
In the three cohort studies, intraoperative blood loss was not significantly different between EBCS and CBCS. The intraoperative blood loss associated with EBCS with a filling method to repair the excised breast volume was significantly less than that associated with CBCS (12). It was assumed to be because of less dissection of the breast tissue.
Complications
Complications associated with EBCS did not frequently occur, but partial wound necrosis and skin flap necrosis occurred in a few cases. Using a wound protector and retaining a thick skin flap might help to prevent them.
Surgical margin
A positive surgical margin rate seen with EBCS was not inferior to that of CBCS in these studies. However, margin involvement varied from 0% to 28.3%. In nine studies, a colored dye was injected into the mammary gland along the resection margin, but the actual practice of presurgical marking and usage of an intraoperative US were poorly shown, except for one study (13). In a recent analysis, data showed that a tumor-free margin distance of 2 mm should be adopted as an adequate excision margin for invasive breast cancer and ductal carcinoma in situ to minimize the risk of local recurrence (15,16).
Oncological outcomes (Tables 5,7)
Full table
Local recurrence
Six studies reported rates of local recurrence. Although the average follow-up time was not enough (12-38.4 months), local recurrence infrequently occurred from 0% to 4.2% (4,8-11,13). According to one study, a local recurrence was more likely to occur with a large tumor (Tis, 0%; T1, 3.7%; T2, 5.1%) (8).
Distant metastasis
Three studies reported the rate of postoperative distant metastases. Possibly because of the short observational period (12-18.1 months) (8,11,13), a distant metastatic lesion was not detected in two studies (11,13). In one case series, the frequency of distant metastases detected over the duration follow-up was related to the tumor size [rate of distant metastases (average follow-up period); Tis, 0% (29.1 months), T1, 3.7% (40 months); T2, 5.1% (39.5 months)] (8).
Overall survival
Five studies reported overall survival. Even though the follow-up period was short, the overall survival was excellent in each study (8-11,13). On the basis of tumor size, one study reported the following result: Tis, 100%; T1, 97.3%; and T2, 95.7% (8). Thus, overall survival seemed to be influenced by tumor size, similar to that of distant metastasis.
Aesthetic estimation and patient satisfaction rating (Tables 5,6)
Aesthetic outcome was estimated in five studies with three methods as follows:
- Four-point scoring system (excellent, good, fair, or poor) (17);
- Five-item-by-4-step method (ABNSW), consisting of five items (Asymmetry, Breast shape, Nipples shape, Skin condition, and Wound scar). Each item was evaluated with four steps (0, poor; 1, fair; 2, good; and 3, excellent). Scores greater than 11 points were considered good or excellent (10);
- Japanese Breast Cancer Society (JBCS) classification, consisting of eight items (breast size, breast shape, breast scar, hardness, nipple/areolar size, shape, nipple/areolar color, nipple position, and inframammary line). Each item was evaluated with a 3-point system (2 points, good; 1 point, fair; and 0 points, poor) or 2-point system (1 point, good; 0 point, poor), and total scores were defined as “excellent” for scores of 11-12, “good” for scores of 8-10, “fair” for scores of 5-7, and “poor” for scores of 0-4 (18).
In three studies, 82.2% to 89.5% patients in the EBCS group were estimated as excellent or good on a 4-point scoring system (6,9,13). In one cohort study, the points for breast scars in the EBCS group were significantly higher than that of the CBCS group (13). The patient satisfaction rating was estimated in five studies (4-6,10,12), and more than 90% of the patients were satisfied with their aesthetic outcome with EBCS in three studies (4,5,10). The satisfaction rating with the surgical scar was high (83.4%) in one study (8) and higher (55.0%) than that of CBCS (31.4%) in another study (12). As for the satisfaction rating for each location, one study with a filling method using Vicryl mesh demonstrated a low satisfaction rating (44.4%) in the outer quadrant than those of the other quadrants (12). However, these surveys were performed less than three years after surgery or radiotherapy.
Discussion
Advantages and disadvantages of EBCS
One advantage of EBCS is its less noticeable scar. However, even if a cancerous breast lesion were resected with a clear margin and a less noticeable scar, the aesthetic outcome would be poor, if the shape of the breast could not be maintained. Thus, the goal of EBCS is not only to perform a curative operation, but also to maintain the postoperative shape of the breast using oncoplastic techniques such as volume displacement or volume replacement.
Disadvantages of EBCS are a longer operative duration, because of its limited small operative field and the additional costs related to the usage of disposable devices. As for the operative duration, a certain amount of learning curve, simplification, and ingenuity with respect to the surgical procedures might be helpful. In one study, the surgeons developed a skin flap using a tumescent technique and could shorten the time in this step of BCS (13). On the other hand, currently, disposable endoscopic devices that are often used for other oncological surgeries are not approved by health insurance providers for breast cancer surgery; thus, usage of these devices may be problematic if EBCS becomes widespread. To improve this issue, in combination with endoscopy, retractors that were not disposable devices were used in five studies for a posterior dissection or for creation of a skin flap of the breast in order to minimize the usage of disposable devices (4,5,8,9,13).
Results from EBCS studies so far
Surgical outcomes
As shown, the overview of the EBCS studies and their surgical outcomes (operative duration, intraoperative blood loss, complication, and surgical margin) seem to be equal to that of CBCS, but these factors vary depending on each operative method (10-13). Therefore, standardization of the operative method should be established.
Oncological outcomes
As for the oncological outcomes (local recurrence, distant metastasis, and overall survival), the EBCS studies suggest an equivalent risk of local recurrence and distant metastasis (4,8-11,13). Therefore, EBCS might not influence the oncological outcomes. Moreover, with EBCS, overall survival also demonstrated favorable results (8-11,13). However, the follow-up durations for the oncological outcomes were within 12-40 months; therefore, it was too short to regard EBCS as comparable to that of CBCS with respect to oncological safety.
Aesthetic estimation and patient satisfaction rating
Most of the EBCS studies demonstrated a favorable aesthetic outcome, and two studies had a better aesthetic outcome than that of CBCS (12,13). In particular, the score for the surgical scar was significantly higher (13), and this result reflected the benefit of EBCS. On the other hand, the patient satisfaction rating was also high and superior to that of CBCS for rating of the surgical scar (12). Nevertheless, the follow-up period for the estimation was not long enough, and the estimation methods for cosmesis, as well as for the patient satisfaction rating, were different among the studies or not clear. Ideally, these kinds of surveys should also be performed at least three years after radiotherapy with standardized and objective methods such as a 4-point scoring system for the aesthetic estimation combined with a European Organisation for Research and Treatment of Cancer (EORTC) breast cancer-specific quality of life questionnaire (EORTC-QLQ-BR23) or Functional Assessment of Cancer Therapy-Breast (FACT-B) for the patient satisfaction rating (19,20).
Key points for the success of EBCS
Patient selection on the basis of clinical examination and breast imaging
One of the most important things for a successful EBCS is the selection of a suitable patient for EBCS. According to the recent studies on oncoplastic breast surgery, when considering a patient for EBCS, the following points should be considered.
Ratio of excised breast tissue volume to the total breast volume
In general, with an excision of up to 20% of the breast volume, some degree of volume displacement by sufficiently undermining the surrounding breast tissue would provide an acceptable cosmetic result (21). It can be achieved in case of EBCS. On the other hand, with an excision of up to 20-40% of the total breast, volume displacement alone may not be sufficient, and therefore, volume replacement by autologous tissue transfer may be necessary (21). In case of EBCS, an LDF is harvested via an axillary fold or a midaxillary incision under endoscopic assistance that could be used for repairing a defect of the outer breast tissue (8,22).
Tumor location
The location of the tumor is also an important factor in the selection of the patient for EBCS. A defect located in the inner or lower pole of the breast is difficult to repair by mobilization via a periareolar incision, an axillary incision, or other small incisions. Thus, if the tumor is located in these two regions, EBCS is not usually recommended, and other oncoplastic breast surgeries via a large enough incision are suitable (13,21).
Glandular density
Glandular density is the third factor in selecting a suitable patient for EBCS. Low-density breast tissue with a major fatty composition has a high risk of fat necrosis after extensive undermining. Therefore, estimation of the breast density based on the Breast imaging Reporting and Data System (BIRADS) that can predict the fatty composition of the breast should be performed (21). This kind of objective estimation determines the possibility of performing a satisfactory EBCS without complications.
For selected patients who would be suitable for EBCS, creating a surgical plan and performing an accurate preoperative assessment regarding the extent of disease is critical for the success of EBCS. MRI is the imaging modality with the highest sensitivity for invasive breast cancer, as well as for ductal carcinoma in situ (DCIS) (23-27), and has a better correlation with the histopathological map concerning cancer extension in the breast (23,24). Actually, a recent study demonstrated that a preoperative MRI could reduce the rate of tumor-positive resection margins and reoperations for BCS (28). In this study, the MRI examination was discussed in detail with a multidisciplinary team of surgeons, pathologists, and radiologists who all specialized in breast cancer. This type of preoperative discussion is recommended for mapping the exact location and true extent of the cancerous lesion within a breast.
Precise presurgical marking
EBCS is performed via a small incision, and the breast lesion is often located far from the incision. Therefore, precise presurgical marking on the breast skin is critical to resect the breast lesion with a suitable margin accurately. Therefore, MRI is an essential modality for EBCS. A problem associated with the use of MRI for presurgical marking for BCS is the deviation of the breast caused by being in a prone position (29). Thus, usually, presurgical marking is performed under second-look US guidance on the basis of MRI findings obtained in the supine position. Using this kind of protocol, one study demonstrated a low rate of positive surgical margins (1.4%, 1/73) (13). Currently, in Japan, because of the shortage of breast radiologists, most breast surgeons learn to read MMGs and breast MRI findings and acquire appropriate skills in breast US. Therefore, on the basis of the diagnostic breast imaging, operating breast surgeons themselves usually perform the preoperative marking and intraoperative marking. Such a situation might be ideal and contribute to low positive margin rates. During the presurgical marking, design for repair of the resected volume is also necessary to reshape the breast. Moreover, to retain blood flow in the remnant breast tissue, identification of the branches passing from the internal thoracic or thoracodorsal vessels is important (Figure 2).
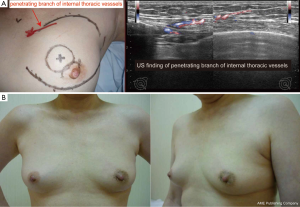
Intraoperative ultrasound scan
Intraoperative marking performed by an intraoperative US scan and a colored dye injection along with the resection line marked on the breast skin is useful for EBCS. Recent studies proved that the intraoperative usage of a US is worthwhile for the identification of tumor localization, achievement of clear margins, and reduction of the re-excision rate (30-32). In addition to these benefits, a reduction in the volume of resected breast tissue was achieved, and thus, the surgical accuracy can enhance the cosmetic outcome of the treated breast (30). The colored dye injected into the breast tissue during EBCS guides the resection of the breast tissue via a small incision.
Accurate resection of a cancer lesion along with intraoperative marking, specimen radiography, and usage of a frozen section analysis
To perform an accurate resection of a cancer lesion with a clear surgical margin, the surgical armamentarium consists of a wound protector, light guided-retractor, and retractor set to lift the skin flap and show the resection line (9,13). To secure the operative view and to protect the skin incision from physical contact, a wound protector is useful. Using these operative devices, an accurate resection based on the intraoperative marking can be performed. Moreover, a specimen radiograph and a frozen section assessment of the margins may contribute to a decrease in the re-excision rate. According to recent studies, a tumor-free margin distance of more than 2 mm is necessary to minimize the risk of a local recurrence (15,16).
Defect repair and reshaping of the treated breast
In most cases in which the excision volume is 20% or less, mobilization of the surrounding breast tissue can be used to repair the defect of the breast during BCS. Ogawa et al. (33) reported that in cases of small dense breasts, in spite of a 30% resection volume, favorable breast shape was obtained using this technique. On the other hand, as for the patients who have a cancerous lesion in the outer upper quadrant and require a large resection of up to 40% or less volume, LDF can be harvested and used for the repair of the defect via an axillary fold incision or a midaxillary incision under endoscopic guidance (8,22). Moreover, if nipple deviation or retraction occurs, it is necessary to undermine the nipple areolar complex (NAC) extensively and perform a round block technique or modified round block technique (34,35). Using these oncoplastic techniques, reshaping of the treated breast can be achieved.
Current issues with EBCS
Until recently, EBCS studies demonstrated technical feasibility and favorable short-term results with respect to the oncological outcomes, aesthetic outcomes, and patient satisfaction ratings. However, there is a lack of long-term follow-up evidence regarding the above-mentioned factors and quality of life (QOL). Moreover, there is no standardized guideline for performing EBCS or general estimation methods for the aesthetic outcomes, patient satisfaction ratings, and QOL in clinical studies. Therefore, under the present circumstances, establishment of standardized guidelines for EBCS and estimation of the postoperative outcomes are urgently needed. Ideally, high-quality comparison studies between EBCS and CBCS should be performed to investigate the feasibility, surgical safety, and aesthetic outcomes and demonstrate the oncological safety, superior patient satisfaction ratings, and QOL.
Prospects of EBCS in the future
EBCS has the advantage of being less noticeable and having a small operative scar, whereas the indication of this method should be limited because of the difficulty in repair of a mammary gland defect via a small incision. On the other hand, recent advances in oncoplastic surgery such as lipofilling (36,37) or a volume replacement technique using an LDF harvested via an axillary incision under endoscopic guidance (22) has the potential to improve the aesthetic outcomes and might expand the indication for EBCS. However, such novel oncoplastic techniques require an adequate number of studies to assess the operative feasibility, long-term oncological outcomes, and aesthetic outcomes.
Conclusions
EBCS has the potential to improve cosmetic outcomes and patient satisfaction for BCS without compromising oncological safety. However, in order to place EBCS as one of the standard breast conservation surgeries, long-term follow-up studies are necessary to investigate oncological outcomes, aesthetic outcomes, and patient satisfaction rating. Moreover, setting clear practice guidelines for the indication, standardized operative procedures, objective assessment methods for aesthetic outcomes, and QOL should be performed promptly.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002;347:1233-41. [PubMed]
- Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 2002;347:1227-32. [PubMed]
- Litière S, Werutsky G, Fentiman IS, et al. Breast conserving therapy versus mastectomy for stage I-II breast cancer: 20 year follow-up of the EORTC 10801 phase 3 randomised trial. Lancet Oncol 2012;13:412-9. [PubMed]
- Tamaki Y, Sakita I, Miyoshi Y, et al. Transareolar endoscopy-assisted partial mastectomy: a preliminary report of six cases. Surg Laparosc Endosc Percutan Tech 2001;11:356-62. [PubMed]
- Owaki T, Yoshinaka H, Ehi K, et al. Endoscopic quadrantectomy for breast cancer with sentinel lymph node navigation via a small axillary incision. Breast 2005;14:57-60. [PubMed]
- Lee EK, Kook SH, Park YL, et al. Endoscopy-assisted breast-conserving surgery for early breast cancer. World J Surg 2006;30:957-64. [PubMed]
- Yamashita K, Shimizu K. Transaxillary retromammary route approach of video-assisted breast surgery enables the inner-side breast cancer to be resected for breast conserving surgery. Am J Surg 2008;196:578-81. [PubMed]
- Nakajima H, Fujiwara I, Mizuta N, et al. Video-assisted skin-sparing breast-conserving surgery for breast cancer and immediate reconstruction with autologous tissue. Ann Surg 2009;249:91-6. [PubMed]
- Saimura M, Mitsuyama S, Anan K, et al. Endoscopy-assisted breast-conserving surgery for early breast cancer. Asian J Endosc Surg 2013;6:203-8. [PubMed]
- Yamashita K, Shimizu K. Endoscopic video-assisted breast surgery: procedures and short-term results. J Nippon Med Sch 2006;73:193-202. [PubMed]
- Park HS, Lee JS, Lee JS, et al. The feasibility of endoscopy-assisted breast conservation surgery for patients with early breast cancer. J Breast Cancer 2011;14:52-7. [PubMed]
- Takemoto N, Koyanagi A, Yamamoto H. Comparison between endoscope-assisted partial mastectomy with filling of dead space using absorbable mesh and conventional conservative method on cosmetic outcome in patients with stage I or II breast cancer. Surg Laparosc Endosc Percutan Tech 2012;22:68-72. [PubMed]
- Ozaki S, Ohara M, Shigematsu H, et al. Technical feasibility and cosmetic advantage of hybrid endoscopy-assisted breast-conserving surgery for breast cancer patients. J Laparoendosc Adv Surg Tech A 2013;23:91-9. [PubMed]
- Sanuki J, Fukuma E, Wadamori K, et al. Volume replacement with polyglycolic acid mesh for correcting breast deformity after endoscopic conservative surgery. Clin Breast Cancer 2005;6:175. [PubMed]
- Behm EC, Beckmann KR, Dahlstrom JE, et al. Surgical margins and risk of locoregional recurrence in invasive breast cancer: an analysis of 10-year data from the Breast Cancer Treatment Quality Assurance Project. Breast 2013;22:839-44. [PubMed]
- Dick AW, Sorbero MS, Ahrendt GM, et al. Comparative effectiveness of ductal carcinoma in situ management and the roles of margins and surgeons. J Natl Cancer Inst 2011;103:92-104. [PubMed]
- Winchester DP, Cox JD. Standards for breast-conservation treatment. CA Cancer J Clin 1992;42:134-62. [PubMed]
- Kijima Y, Yoshinaka H, Funasako Y, et al. Immediate breast reconstruction using autologous free dermal fat grafts provides better cosmetic results for patients with upper inner cancerous lesions. Surg Today 2011;41:477-89. [PubMed]
- Sprangers MA, Groenvold M, Arraras JI, et al. The European Organization for Research and Treatment of Cancer breast cancer-specific quality-of-life questionnaire module: first results from a three-country field study. J Clin Oncol 1996;14:2756-68. [PubMed]
- Brady MJ, Cella DF, Mo F, et al. Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. J Clin Oncol 1997;15:974-86. [PubMed]
- Clough KB, Kaufman GJ, Nos C, et al. Improving breast cancer surgery: a classification and quadrant per quadrant atlas for oncoplastic surgery. Ann Surg Oncol 2010;17:1375-91. [PubMed]
- Serra-Renom JM, Serra-Mestre JM, Martinez L, et al. Endoscopic reconstruction of partial mastectomy defects using latissimus dorsi muscle flap without causing scars on the back. Aesthetic Plast Surg 2013;37:941-9. [PubMed]
- Boetes C, Mus RD, Holland R, et al. Breast tumors: comparative accuracy of MR imaging relative to mammography and US for demonstrating extent. Radiology 1995;197:743-7. [PubMed]
- Amano G, Ohuchi N, Ishibashi T, et al. Correlation of three-dimensional magnetic resonance imaging with precise histopathological map concerning carcinoma extension in the breast. Breast Cancer Res Treat 2000;60:43-55. [PubMed]
- Esserman L, Hylton N, Yassa L, et al. Utility of magnetic resonance imaging in the management of breast cancer: evidence for improved preoperative staging. J Clin Oncol 1999;17:110-9. [PubMed]
- Schouten van der Velden AP, Schlooz-Vries MS, Boetes C, et al. Magnetic resonance imaging of ductal carcinoma in situ: what is its clinical application? A review. Am J Surg 2009;198:262-9. [PubMed]
- Tozaki M. Diagnosis of breast cancer: MDCT versus MRI. Breast Cancer 2008;15:205-11. [PubMed]
- Obdeijn IM, Tilanus-Linthorst MM, Spronk S, et al. Preoperative breast MRI can reduce the rate of tumor-positive resection margins and reoperations in patients undergoing breast-conserving surgery. AJR Am J Roentgenol 2013;200:304-10. [PubMed]
- Turnbull L, Brown S, Harvey I, et al. Comparative effectiveness of MRI in breast cancer (COMICE) trial: a randomised controlled trial. Lancet 2010;375:563-71. [PubMed]
- Krekel NM, Haloua MH, Lopes Cardozo AM, et al. Intraoperative ultrasound guidance for palpable breast cancer excision (COBALT trial): a multicentre, randomised controlled trial. Lancet Oncol 2013;14:48-54. [PubMed]
- Haloua MH, Krekel NM, Coupé VM, et al. Ultrasound-guided surgery for palpable breast cancer is cost-saving: results of a cost-benefit analysis. Breast 2013;22:238-43. [PubMed]
- Yu CC, Chiang KC, Kuo WL, et al. Low re-excision rate for positive margins in patients treated with ultrasound-guided breast-conserving surgery. Breast 2013;22:698-702. [PubMed]
- Ogawa T, Hanamura N, Yamashita M, et al. Breast-volume displacement using an extended glandular flap for small dense breasts. Plast Surg Int 2011;2011:359842.
- Ogawa T. Usefulness of breast-conserving surgery using the round block technique or modified round block technique in Japanese females. Asian J Surg 2014;37:8-14. [PubMed]
- Zaha H, Onomura M, Unesoko M. A new scarless oncoplastic breast-conserving surgery: modified round block technique. Breast 2013;22:1184-8. [PubMed]
- Rosing JH, Wong G, Wong MS, et al. Autologous fat grafting for primary breast augmentation: a systematic review. Aesthetic Plast Surg 2011;35:882-90. [PubMed]
- Claro F Jr, Figueiredo JC, Zampar AG, et al. Applicability and safety of autologous fat for reconstruction of the breast. Br J Surg 2012;99:768-80. [PubMed]


