Cryosurgery for pancreatic cancer
Introduction
Pancreatic cancer is the main cause of cancer-related mortality becoming such a major worldwide public health problem. Long-term survival remains poor with less than 5% of patients alive 5 years after diagnosis (1). The radical surgical resection represents the only chance for cure but unfortunately is possible in only 5-25% of cases; the 5-year survival rates for the most favorable patients who undergo resection and adjuvant therapy do not exceed 29% (2). Most patients present with unresectable pancreatic cancer and 40-45% of them have metastatic disease, mainly hepatic, which carries a median survival of 3 to 6 months.
The treatment of patients with advanced disease remains palliative. For patients with unresectable pancreatic cancer, radiation therapy is most often used to relieve painful disease sites, while chemotherapy is prescribed to reduce the rate of tumor growth and thus prolong surviva (2-4). However, as a whole, the effect of recent treatment for pancreatic cancer is pessimistic. According to the American Cancer Society, for all stages of pancreatic cancer combined, the one-year relative survival rate is 20%, and the five-year rate is 4%. Therefore, it is necessary to seek for novel modality (5).
Local ablative therapies have established role in the management of cancers of other organs. Delicate nature of the pancreatic parenchyma and the risk of injury to important structures such as duodenum or common bile duct in the proximity of the organ have been the main limiting factors in application of these ablative techniques to the pancreas (6,7).
Early in 1970 Myers pointed out the possible use of pancreatic cryosurgery in primate experiment. In 1991 Patiutko et al. (8) reported use of cryosurgery in combination with radiation for locally advanced pancreatic cancer. In 2002, Kovach et al. (9) performed phase I clinical trial of use of cryosurgery for treatment of pancreatic cancer. Korpan (10,11) stated that that almost all patients with pancreatic cancer could undergo cryosurgery with no major complication. It is Fuda Cancer Hospital Guangzhou, China, where authors are working from 2004, we started use of cryosurgery for treatment of locally advanced pancreatic cancer. At the beginning, the cryosurgery has been performed intraoperatively, and from 2005, the percutaneous technique becomes the main mode of cryosurgery for the treatment of pancreatic cancer at this hospital (12). Up to now a total 265 patients with pancreatic cancer, most of whom have stage III or stage IV of disease, underwent a total of 322 times of cryoablation alone or in combination with other therapies.
Indication
Cryosurgery is considered to attain the following aims:
Palliation of symptoms, in particular pain-relief;
Treatment of locally advanced disease;
Benefit of survival.
Theoretically, as for other cancer, cryosurgery is confined to treatment of unresectable pancreatic cancer. As a matter of fact, there are few patients with pancreatic carcinoma who can be resected by operation, therefore, majority of pancreatic cancer should be treated with cryosurgery or cryosurgery in combination with pancreatic resection, if there is no contraindication (10,11).
Technology
The procedure of cryosurgery is performed with intraoperative or percutaneous approaches.
Intraoperative cryosurgery
Before the surgical procedure, patients are administrated a general anaesthesia and positioned for an upper abdominal incision. The involved pancreas is exposed by trans-peritoneal mobilization of the bowel and stomach. Once the specific pancreatic mass is identified, an 18-gauge Tru-Cut biopsy needle is used to obtain one to two cores of tissue from solid mass. If it is discovered that the tumor is unresectable through a thorough investigation, cryosurgery is performed in direct vision and under the guidance of ultrasound. A variable number (one to three) of 2- or 3-mm cryoprobes are placed directly into the pancreatic mass and positioned under ultrasound guidance. Generally, lesions smaller than 3 cm could be reliably frozen with a single, centrally placed, 3-mm probe, and large lesions require multiple probes. A double cycle of freeze/thaw procedure is used with an argon-gas-based cryosurgical unit (Endocare, Inc., CA, USA). Each cryoprobe is cooled to –160 °C and the resulting iceball monitored with ultrasound until frozen region encompass the entire mass of the tumor with at least a “0.5-cm safe border”. The tissue is then allowed to slowly thaw to 0 °C. A second cycle of freezing/thawing is performed after any necessary repositioning of the cryoprobes. After the freezing process is completed, the cryoprobes are removed and the still-frozen tract made by the cryoprobe is packed with thrombin-soated Gelfoam to control bleeding. For the metastases of the liver, the cryosurgery is performed simultaneously (13).
Percutaneous cryosurgery
Patients fast for 24 hrs prior to the operation, and oral laxatives are given 12 hrs before the procedure. Pancreatic secretion is inhibited by medication 24 hrs before the operation to reduce the rates of complications. Sufficient breath training is given to ensure steady breath movement during the procedure. For patients with obstructive jaundice, percutaneous transhepatic or endoscopic cholangiodrainage is performed in order to relieve symptoms, improve liver function, and reduce the pancreatic edema.
The patient is positioned so that the whole lesion was seen, with ultrasound performed through the abdomen or back. Conventional abdominal ultrasonography and/or CT is utilized to target the tumor and to identify the probe entry site. Three possible transabdominal approaches were conducted, including approaching through stomach (in most cases) or left lobe of liver, approaching between stomach and transverse colon by pressing with ultrasound probe in case of large tumors. Transdorsal approach was carried out as approaching between T12 and L1, 4-7 cm away from spine on the left side.
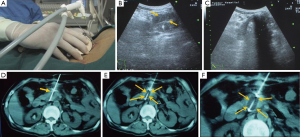
Patients receive general anesthesia or local anesthesia with 1% lidocaine for the cryoablation procedure. A 1.7-mm cryoprobe is advanced into the center of the mass under real-time CT and/or ultrasound guidance through transperitoneal or retroperitoneal access. The probe is advanced through the center of the mass until the tips are positioned along its distal inner border. For tumors larger than 3 cm in maximum diameter 2 to 4 1.7-mm cryoprobes with argon-based cryosurgical system (Endocare, CA, USA) are used. Cryoprobes are placed 1 cm apart from one another in the tumor. The tip of the cryoprobe is cooled to –160 °C and ice ball growth was monitored by CT and/or real-time sonography. The leading edge of the ice ball is seen under ultrasound as an echogenic interface with strong posterior acoustic shadowing until the frozen region encompassed the entire tumor with a “0.5-cm safety border”. Two freeze cycles are applied for an average of 5 minutes each, followed by a 10 minutes of active thawing. The coverage of ice-ball was confirmed along with ultrasound and CT monitoring (Figure 1). For liver metastases, simultaneous cryoablation with 2 cycles of freezing/thawing, is performed using additional cryoprobes which were inserted through the right intercostal space (11,14). In contrast to pancreatic cryoablation, hepatic freezing time is 10 min. During the puncture, involvement of the bowel generally does not result in substantial complications. However, a safer approach is achieved by transversing the stomach. Intestine and colon should be avoided especially when using large-diameter cryoprobe. After the freezing process is completed, the cryoprobe is removed and entry site is packed with thrombin-soaked Gelfoam to control bleeding.
Postoperative management
All patients should be admitted into the intensive care unit (ICU) for monitoring. Somatostatin analogue (Octreotide acetate) is given via intravenous infusion for 3 days, or until 12-24 hrs after disappearance of abdominal pain. Serum amylase is detected during preoperative assessment 1 to 7 days postoperatively or until the enzyme activity returned to within normal range. The patients are evaluated using CT or PET-CT every 4 to 6 weeks after cryoablation. Once the local recurrence of the tumor in pancreas and liver, repeat cryoablation may be performed (11,12).
In pancreatic tumors (especially of the head of pancreas), it is prudent to perform palliative bypass with procedures such as endoscopic stent placement or cholecystojejunostomy and gastrojejunostomy before or after cryoablation.
Clinical results
There are few of reports about cryosurgery for treatment of pancreatic cancer. Kovach (9) reported that 9 patients with unresectable pancreatic cancer underwent a total of 10 sessions of intraoperative cryosurgery under ultrasound guidance. There was no cryosurgery-related motality and no post-cryosurgery pancreatic fistulae and pancreatitis. Following the treatment, patients had alleviation of pain and decrease of analgesic dose. All patients could take normal diet at discharge from hospital. Patiutko et al. (8) treated 30 patients with locally advanced pancreatic cancer with combination of cryosurgery and radiation. All patients had effective control of pain, decrease of CA 19-9, improvement of performance, increased survival rates. Korpan (10) summarized the experience of cryosurgery for pancreatic cancer, and showed that there was a good efficacy of the modality for most of the patients.
Seventy-three consecutive patients with pancreatic cancer were admitted to Fuda Hospital Guangzhou from Sept. 2008 to Sept. 2009. Of 59 patients, 35 males and 24 females, with a median age of 57 years (range, 38 to 85 years), underwent percutaneous cryoablation (14). In the prospective study, enrolled patients fitted the following criteria: (I) after multi-disciplinary consultation, their tumor was considered unresectable due to large size, involvement of superior mesenteric artery and/or celiac trunk, or multiple enlarged retroperitoneal lymph node; (II) although there were liver metastases, nodules were less than 3 in number and less than 5 cm in diameter; (III) there was better general status of health; (IV) no multiple metastases apart from liver, and no severe dysfunction of liver and kidneys, and no coagulation abnormality was detected; and (V) patient totally refused surgical intervention. There were a total of 76 biopsy-proven tumors in 59 patients, located at the pancreas head (56 cases), body (7 cases), and tail (13 cases). The median longest diameter of tumor on CT scan was 4.5 cm (range, 3.1-6.3 cm). Nineteen patients had liver metastases. Fifty-four patients (91.5% of all cases) had received 5-FU- or gemcitabine-based chemotherapy prior to administration to this hospital with no response or an unsuccessful outcome. The results are followed.
Response
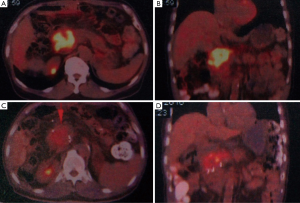
The median hospital stay was 21 days, and a median follow-up was 7.5 months (range 3 to 14 months). According to WHO protocol, 2 patients (3.4%) achieved complete response (CR) in pancreatic cancer (Figure 2), 23 (39.0%) had partial response (PR), 30 (50.8%) had stable disease (SD), 4 (6.8%) had progressive disease (PD).
Survival
The survival of 59 patients with pancreatic cancer underwent percutaneous cryosurgery is seen in Table 1. The median survival was 8.4 months. The overall survival at 3, 6 and 12 months was 89.7%, 61.1% and 34.5%, respectively. Of 40 patients without liver metastases and 19 patients with liver metastases, 3-, 6-, and 12-month survival were 92.4%, 84.2%, and 62.1% and 59.3%, 43.2%, and 13.7%, respectively (P<0.05).

Full Table
Adverse effects and complication
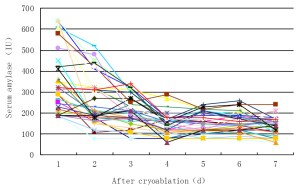
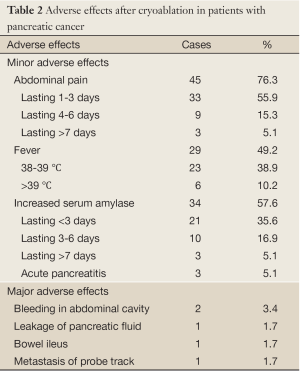
There were no deaths associated with cryoablation. The adverse effects were seen in Table 2. Minor adverse effects included transient abdominal pain, which was seen in 45 cases (59.2%) and lasted 1-3 days in 33 cases (55.9%), 4-7 days in 9 (16.1%) and 8-11 days in 3 (5.1%). Fever was seen in 29 cases (49.2%), in whom 6 cases (10.2%) had temperature more than 39 °C. The fever generally lasted 3-4 days and returned to within normal range within 7 days in all cases. The elevation of serum amylase was seen in 34 (44.7%). The enzyme levels had mildly increased in the most of the cases; more than 3 times of the normal upper limit (reference value <180 IU/dL) was only seen in 6 cases (17.6%). The increased enzyme levels returned to normal range within 3days in 22 cases (84.7%) (Figure 3). Acute pancreatitis with abdominal pain and increased amylase levels was diagnosed in 3 cases (5.1%) and was classified as a mild type. All 3 cases of pancreatitis were cured. Major morbidity was intra-abdominal bleeding in 2 cases (3.4%), which presented within 24 hrs after completing the cryoablation. The bloody liquid, 600 mL and 1,100 mL, respectively, was drained from the abdominal cavity. One patient received a transfusion of fresh blood of 400 mL. The bleeding ceased within 3 d in all 2 patients.
Full Table
There was one patient who had pancreatic leakage with amylase-rich (2,100 IU/dL) increasing ascites, and was given supportive care, abdominal drainage, use of proton pump inhibitor and increase of dose infused octreotide acetate. As a result, the amount of drained abdominal fluid gradually decreased after 3 days and disappeared after 8 days, with no serious consequences. One case was complicated by bowel ileus and underwent nasogastric tube and fluid resuscitation. The symptoms of the ileus were relieved after 48 hrs later. No cause of the ileus was detected. One case was discovered to have a mass under the abdominal wall measuring 3 cm × 2.5 cm in size on PET-CT. It was confirmed to be adenocarcinoma and was considered as metastasis in the cryoprobe tract, and was given percutaneous cryoablation. One month later, the follow-up with PET-CT showed that the tumor activity had disappeared.
Discussion
Local ablation for pancreatic cancer
A computerized search was made of the MEDLINE database and Pubmed, and revealed 13 reports but ours describing local ablative therapies used for the treatment of pancreatic cancer in humans (Table 3). Siriwardena group from Manchester developed optimal thermal kinetic parameters (28) in a previously validated porcine model (29) and used these parameters for RFA of advanced pancreatic cancer. Bown’s group from London had published sequential animal studies since 1992 (30) which eventually resulted in Phase I clinical trial in 2002. In this trial the authors described successful use of photodynamic therapy (PDT) in 16 patients of pancreatic cancer using meso-tetrahydroxyphenyl chlorine. Four patients received repeat treatment, and one of them had it repeated third time. High-intensity focused ultrasound (HIFU) ablation was used for the ablation of advanced pancreatic cancer in eight patients (19). Although the current literature on electrolytic ablation is mainly in the form of animal studies (31-33) from Maddern’s study electrolysis appears to have the potential to be adapted for pancreatic tissue ablation.
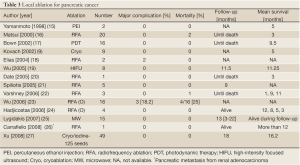
Full Table
From above described, the generators, probes, and ablation techniques used in these reports showed marked variation. Inasmuch as the ablative techniques used in these studies were different, the results of these studies cannot be compared or added. The important lessons can however be learned by sharing the experiences of complications and treatment outcomes.
The local selective destruction of solid tumors, especially liver tumors, by cryoablation is now well-established (11,34). In the liver, the larger volume of normal hepatic parenchyma will surround tumor-bearing tissue. In contrast, the smaller volume of the pancreatic gland, the fragile pancreatic parenchyma and its proximity to structures as the stomach, duodenum, colon, common bile duct, and vessels are the reasons that may explain why cryoablation has not yet been widely used for pancreatic tumors.
Use of cryosurgery for the treatment of pancreatic cancer is based on the following consideration:
-
Low temperature less than –40 °C induces definite destruction area of targeted tissue. Experimental cryoablation of normal pancreas of pigs, showed hyperemia, edema or hemorrhage in the unfrozen parts of the pancreas. Histological assessment revealed a significant level of necrosis in the central and lateral regions of the tissue frozen within the ice-ball. All cellular ultrastructure was destroyed and only observable as a few of remaining nuclei with broken crests and degranulated mitochondria and rough endoplasmic reticulum (35);
-
At laparotomy the pancreatic cancer is discovered to be unresectable, the conventional management is bypass operation without intervention for the tumor (9);
-
The cryosurgery may make up the shortcoming of the conventional therapy, and the operation, therefore, become “radical” from “palliative”;
-
According to the experience of percutaneous cryoablation for liver tumors, the freezing hardly injures neighboring organs (36). Large vessels in the vicinity of the pancreas have the effect of a heat sinks effect, i.e. warming effect of blood flowing by which counteracts the cryodestruction, therefore, pancreatic freezing hardly induces large bleeding unless the probe directly penetrates the vessels (37,38);
-
It is presumed that cryosurgery for pancreatic lesion may be reasonable. The study on RFA shows that poor blood flow in such tumors causes to retain heat, It is known that the blood supply to pancreas is much less than that to liver (39), therefore, hypovascular pancreatic cancers are considered to be good targets for therm-based treatment. The phenomenon may be adaptable for cryoablation;
-
It is reported that tumor-reduction surgery improves the prognosis of patients with local advanced pancreatic cancer with evidence of metastases (stage III), even if there are cancer cells remaining at the surgical resection margins (40). To a great extent, the purpose of the treatment for pancreatic cancer is to debulk the tumor rather than “radical treatment”. Cryoablation is adaptable to this goal;
-
It is reported that the cryoablated cancerous tissue has an increased sensitivity to chemo/radiotherapy (41);
-
Cryoablation-induced immunity (which we call “cryoimmunity”) may play a role in the control or eradication of the residue cancerous cells (42,43) that is in contrast to other heat-based ablation techniques.
Theoretically, intraoperative cryosurgery has the advantage of protecting the adjacent viscerals by cooling the area, to perform the ablation under direct vision, however, further attempt discovers that the procedure percutaneously is safely performed. According to our experience, the image of structures which emerges on CT or ultrasound during percutaneous cryoablation, sometimes, is more clear that that which are seen at laparotomy.
Therapeutic efficacy
Our results of percutaneous cryosurgery for 59 patients with unresectable advanced pancreatic cancer showed that at 4 months after treatment, most patients showed the objective response of tumor with PR 38.8% and SD 54.2%, and the overall survival at 3, 6 and 12 months were 89.7%, 61.1% and 34.5%, respectively.
The data of cryosurgery for pancreatic cancer are too few to comment its efficacy. However, if the poor prognosis of advanced pancreatic cancer and few option are considered, the current figure are encouraging though larger series of cases are needed to secure the results.
As said in “Introduction” before, the treatment of patients with advanced disease remains pessimistic. Both ECOG trial and GITSG studies showed the median survival is 10 and 8.3 months, respectively, in patients receiving 5-FU-based chemoradiation (44). It is evident that the patients with locally advanced, unresectable pancreatic cancer do not benefit from gemcitabine-based chemotherapy (2). There are several combined therapies used in an attempt to improve survival of patients with locally advanced disease. However, the results are disappointing as well. Kindler (45) treated patients with previously untreated advanced pancreatic cancer with gemcitabine and Bevacizumab, and results showed that the 6-month survival rate was 77%, median survival was 8.8 months. Kulke et al. (46) reported that 259 patients with metastatic pancreatic cancer received gemcitabine at a fixed dose rate or in combination with cisplatin, docetaxel, or irinotecan; the overall survival time was 6.4 to 7.1 months. Ardavanis et al. (47) reported that use of biweekly gemcitabine in combination with erlotinib for advanced pancreatic cancer enhanced the median overall survival and time to progression at the time of assessment of 7.5 and 5.5 months, respectively. Our results are comparable to above-mentioned chemoradiotherapy for unresectable locally advanced pancreatic cancer. It is noted that most of our patients (91.5%) have received 5-FU- or gemcitabine-based chemotherapy without response. In terms of their chemotherapy-refractory response, the cryosurgery is promising.
Safeness of cryosurgery for pancreas
A great attention is paid for the safety of cryosurgery of pancreatic cancer. The safeness may be enlightened from heat-based ablation (see Table 3). Matsui et al. (16), reported 20 patients with unresectable pancreatic adenocarcinoma treated with RFA. Two patients (10%) died from critical complications. The authors consider that the method is relatively safe and could be used to treat unresectable tumors without metastasis or patients with benign pancreatic tumors such as insulinomas and glucagonomas. Goldberg et al. (48) in a porcine model trial concluded that endoscopic ultrasound-guided RFA can be used safely to create discrete zones of coagulation necrosis in the porcine pancreas. Elias et al. (18) reported their experience in two patients with pancreatic metastatic tumors (from renal cancer), who died from severe post RFA necrotizing pancreatitis, and concluded that because of severe complications, RFA in the pancreas is not recommended.
Korpan (10) made an experimental study on dogs that received pancreatic cryosurgery with the disc cryoprobe. No animal developed cryosurgery-related mortality and complications. No post-cryosurgery bleeding, pancreatic fistulae or secondary infection were observed. In our series, there was no cryosurgery-related mortality.
Chen et al. (49) observe the consequence of cryosurgery for pancreas of healthy pigs. Two groups of animals underwent mild and deep (–170 °C) hypothermic freezing. It is discovered that mild hypothermic cryosurgery with liquid nitrogen superficial refrigeration might lead to pancreatic injury and induce acute pancreatitis, yet deep hypothermic cryosurgery with adequate time showed a promising effect in destroying pancreatic tissue and preventing acute pancreatitis.
Chiu et al. (35) performed biochemical analysis and histological assessment for pancreatic cryoablation in healthy pigs with an argon-helium cryoprobe. There was a significant increase of serum amylase levels for a brief period. However, the enzyme levels also increased in the control group. All experimental pigs appeared healthy until the sacrifice time. Our conclusion is that cryosurgery is a safe and effective ablative procedure for pancreatic tissue resulting in minimal complications.
In our 59 patients with pancreatic cancer, no deaths associated with cryoablation were observed. Most of adverse effects are minor, including transient abdominal pain and increased serum amylase, major morbidity including intra-abdominal bleeding pancreatic leaks, ileus, and metastasis of the probe track in 5 cases, without severe outcome after proper management. It is suggested that the risk of cryosurgery for pancreatic cancer is fewer than that which is conceived.
For decrease of adverse effect, especially major complication of pancreatic cryosurgery, it is suggested to improve the procedure. Experimental study showed that freezing of pancreatic tissue for 5 or 10 mins appeared a similar outcome from the microscopic and electron microscopic examination of the targeted tissue (35). Therefore, for pancreatic tumors freezing time is 5-8 mins per cycle, in contrast to cryoablation in the liver, where freeze cycles last for 10-15 mins (41). This is anticipated to decrease the major adverse effects. In addition, it is enphasized to use of the ultrafine 1.7-mm cryoprobe, which hardly results in parenchymal rupture even when it penetrates organs such as the stomach or the bowel.
The acute necrotizing pancreatitis was reported as severe complications of RFA (18), but it, in fact, is not a common postoperative complication. For prevention of this complication, postoperative use of pancreas-suppressive drug, such as somatostatin analogue is necessary.
Conclusions
Patients with pancreatic cancer have a dismal prognosis. New treatment modalities are thus urgently needed. From current data and our initial experience, cryoablation appears to be a feasible, potentially safe and promising option in patients with locally advanced and unresectable pancreatic cancer. As Korpan pointed out that there are almost no known contraindications to the use of cryosurgery for pancreatic cancer. For most patients with pancreatic cancer, cryosurgery can substitute conventional surgery. These observations need to be confirmed by more studies. We now apply cryoablation as the standard approach in patients with unresectable pancreatic cancer. Nevertheless, further research in a greater number of cases is needed to support the encouraging results.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Ducreux M, Boige V, Malka D. Treatment of advanced pancreatic cancer. Semin Oncol 2007;34:S25-30.
- Wilkowski R, Thoma M, Bruns C, et al. Chemoradiotherapy with gemcitabine and continuous 5-FU in patients with primary inoperable pancreatic cancer. JOP 2006;7:349-60.
- Eickhoff A, Martin W, Hartmann D, et al. A phase I/II multicentric trial of gemcitabine and epirubicin in patients with advanced pancreatic carcinoma. Br J Cancer 2006;94:1572-4.
- Wada K, Takada T, Amano H, et al. Trend in the management of pancreatic adenocarcinoma--Japan vs. US and Europe. Nihon Geka Gakkai Zasshi 2006;107:187-91.
- Claude L, Mornex F. Chemoradiation in pancreatic carcinoma. Cancer Radiother 2003;7:254-65.
- Myers RS, Hammond WG, Ketcham AS. Cryosurgery of primate pancreas. Cancer 1970;25:411-4.
- Date RS. Current status of local ablative techniques in the treatment of pancreatic cancer. Pancreas 2006;33:198-9.
- Patiutko IuI, Barkanov AI, Kholikov TK, et al. The combined treatment of locally disseminated pancreatic cancer using cryosurgery. Vopr Onkol 1991;37:695-700.
- Kovach SJ, Hendrickson RJ, Cappadona CR, et al. Cryoablation of unresectable pancreatic cancer. Surgery 2002;131:463-4.
- Korpan NN. Hepatic cryosurgery for liver metastases. Long-term follow-up. Ann Surg 1997;225:193-201.
- Korpan NN. Cryosurgery: ultrastructural changes in pancreas tissue after low temperature exposure. Technol Cancer Res Treat 2007;6:59-67.
- Xu KC, Niu LZ. Cryosurgery for Cancer. In: Xu KC, Niu LZ. eds. Pancreatic cancer. Shanghai: Shanghai Science-Technology-Education Pub., 2007:234-45.
- Xu KC, Niu LZ, Hu YZ, et al. Cryosurgery with combination of (125)iodine seed implantation for the treatment of locally advanced pancreatic cancer. J Dig Dis 2008;9:32-40.
- Kulenović A, Sarac-Hadzihalilović A. Blood vessels distribution in body and tail of pancreas- a comparative study of age related variation. Bosn J Basic Med Sci 2010;10:89-93.
- Yamamoto S, Miyake I, Takatori K, et al. Percutaneous ethanol injection for unresectable pancreatic cancer-report of two cases Gan To Kagaku Ryoho 1998;25:1969-71.
- Matsui Y, Nakagawa A, Kamiyama Y, et al. Selective thermocoagulation of unresectable pancreatic cancers by using radiofrequency capacitive heating. Pancreas 2000;20:14-20.
- Bown SG, Rogowska AZ, Whitelaw DE, et al. Photodynamic therapy for cancer of the pancreas. Gut 2002;50:549-57.
- Elias D, Baton O, Sideris L, et al. Necrotizing pancreatitis after radiofrequency destruction of pancreatic tumours. Eur J Surg Oncol 2004;30:85-7.
- Wu F, Wang ZB, Zhu H, et al. Feasibility of US-guided high-intensity focused ultrasound treatment in patients with advanced pancreatic cancer: initial experience. Radiology 2005;236:1034-40.
- Date RS, Biggins J, Paterson I, et al. Development and validation of an experimental model for the assessment of radiofrequency ablation of pancreatic parenchyma. Pancreas 2005;30:266-71.
- Spiliotis J, Datsis A, Chatzikostas P, et al. Pancreatic cancer palliation using radiofrequency ablation. A new technique, Cancer Ther 2005;3:379-82.
- Varshney S, Sewkani A, Sharma S, et al. Radiofrequency ablation of unresectable pancreatic carcinoma: feasibility, efficacy and safety. JOP 2006;7:74-8.
- Wu Y, Tang Z, Fang H, et al. High operative risk of cool-tip radiofrequency ablation for unresectable pancreatic head cancer. J Surg Oncol 2006;94:392-5.
- Hadjicostas P, Malakounides N, Varianos C, et al. Radiofrequency ablation in pancreatic cancer. HPB (Oxford) 2006;8:61-4.
- Lygidakis NJ, Sharma SK, Papastratis P, et al. Microwave ablation in locally advanced pancreatic carcinoma--a new look Hepatogastroenterology 2007;54:1305-10.
- Carrafiello G, Laganà D, Recaldini C, et al. Radiofrequency ablation of a pancreatic metastasis from renal cell carcinoma: case report. Surg Laparosc Endosc Percutan Tech 2008;18:64-6.
- Xu KC, Niu LZ, He WB, et al. Percutaneous cryosurgery for the treatment of hepatic colorectal metastases. World J Gastroenterol 2008;14:1430-6.
- Date RS, McMahon RF, Siriwardena AK. Radiofrequency ablation of the pancreas. I: Definition of optimal thermal kinetic parameters and the effect of simulated portal venous circulation in an ex-vivo porcine model. JOP 2005;6:581-7.
- Chatlani PT, Nuutinen PJ, Toda N, et al. Selective necrosis in hamster pancreatic tumours using photodynamic therapy with phthalocyanine photosensitization. Br J Surg 1992;79:786-90.
- Mikvy P, Messman H, MacRobert AJ, et al. Photodynamic therapy of a transplanted pancreatic cancer model using meta-tetrahydroxyphenylchlorin (mTHPC). Br J Cancer 1997;76:713-8.
- Morrison CP, Teague BD, Court FG, et al. Experimental studies of serum cytokine concentration following pancreatic electrolytic ablation. Med Sci Monit 2003;9:BR43-6.
- Morrison CP, Court FG, Wemyss-Holden SA, et al. Perductal electrolytic ablation of the porcine pancreas: a minimally invasive option-studies of morbidity and mortality. Surg Endosc 2004;18:1435-41.
- Morrison CP, Court FG, Teague BD, et al. Endoscopic perductal electrolytic ablation of the pancreas: experimental studies of morbidity and mortality. Dig Dis 2005;23:83-91.
- Xu KC, Niu LZ, Hu YZ, et al. A pilot study on combination of cryosurgery and (125)iodine seed implantation for treatment of locally advanced pancreatic cancer. World J Gastroenterol 2008;14:1603-11.
- Chiu D, Niu L, Mu F, et al. The experimental study for efficacy and safety of pancreatic cryosurgery. Cryobiology 2010;60:281-6.
- Hoffmann NE, Bischof JC. The cryobiology of cryosurgical injury. Urology 2002;60:40-9.
- Lagerveld BW, van Horssen P, Pes MP, et al. Immediate effect of kidney cryoablation on renal arterial structure in a porcine model studied by imaging cryomicrotome. J Urol 2010;183:1221-6.
- Yu HB, Ge CL, Huang ZH, et al. Effect of targeted argon-helium cryoablation on the portal region in canine livers. Nan Fang Yi Ke Da Xue Xue Bao 2009;29:538-40.
- Perez-Johnston R, Lenhart DK, Sahani DV. CT angiography of the hepatic and pancreatic circulation. Radiol Clin North Am 2010;48:311-30, viii.
- Lillemoe KD, Cameron JL, Yeo CJ, et al. Pancreaticoduodenectomy. Does it have a role in the palliation of pancreatic cancer? Ann Surg 1996;223:718-25; discussion 725-8.
- Gage AA, Baust JM, Baust JG. Experimental cryosurgery investigations in vivo. Cryobiology 2009;59:229-43.
- Joosten JJ, Muijen GN, Wobbes T, et al. In vivo destruction of tumor tissue by cryoablation can induce inhibition of secondary tumor growth: an experimental study.Cryobiology 2001;42:49-58.
- Sabel MS. Cryo-immunology: a review of the literature and proposed mechanisms for stimulatory versus suppressive immune responses. Cryobiology 2009;58:1-11.
- Bast BC, Kuff DW, Pollock RE. Holland-Frei Cancer Medicine, eds. In: Wolff RT, Abbruzzese JL, Evans DB. eds. Neoplasm of the Exocrine Pancreas. Harcourt: Asia Pte. Ltd., 2000:1437-64.
- Kindler HL, Friberg G, Singh DA, et al. Phase II trial of bevacizumab plus gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol 2005;23:8033-40.
- Kulke MH, Tempero MA, Niedzwiecki D, et al. Randomized phase II study of gemcitabine administered at a fixed dose rate or in combination with cisplatin, docetaxel, or irinotecan in patients with metastatic pancreatic cancer: CALGB 89904. J Clin Oncol 2009;27:5506-12.
- Ardavanis A, Kountourakis P, Karagiannis A, et al. Biweekly gemcitabine (GEM) in combination with erlotinib (ERL): an active and convenient regimen for advanced pancreatic cancer. Anticancer Res 2009;29:5211-7.
- Goldberg SN, Mallery S, Gazelle GS, et al. EUS-guided radiofrequency ablation in the pancreas: results in a porcine model. Gastrointest Endosc 1999;50:392-401.
- Chen XL, Ma Y, Wan Y, et al. Experimental study of the safety of pancreas cryosurgery: the comparison of 2 different techniques of cryosurgery. Pancreas 2010;39:92-6.